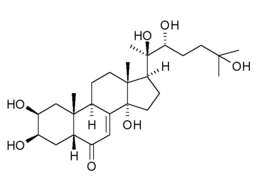
20-Hydroxyecdysone
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Biological half-life | 4-9 hours |
| Excretion | Urinary:?% |
| Identifiers | |
| |
| CAS Number |
5289-74-7 |
| PubChem (CID) | 5459840 |
| ChemSpider |
4573597 |
| ChEBI |
CHEBI:16587 |
| ChEMBL |
CHEMBL224128 |
| Chemical and physical data | |
| Formula | C27H44O7 |
| Molar mass | 480.63 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
20-Hydroxyecdysone (ecdysterone or 20E) is a naturally occurring ecdysteroid hormone which controls the ecdysis (moulting) and metamorphosis of arthropods. It is therefore one of the most common moulting hormones in insects, crabs, etc. It is also a phytoecdysteroid produced by various plants, including Cyanotis vaga, where its purpose is presumably to disrupt the development and reproduction of insect pests. In arthropods, 20-hydroxyecdysone acts through the ecdysone receptor. Although mammals lack this receptor, 20-hydroxyecdysone may affect mammalian (including human) biological systems in vitro, but there is uncertainty whether any in vivo or physiological effects occur. 20-Hydroxyecdysone is an ingredient of some supplements that aim to enhance physical performance, but there is no clinical evidence for this effect.[1]
Sources in arthropods
The primary sources of 20-hydroxyecdysone in larvae are the prothoracic gland, ring gland, gut, and fat bodies. These tissues convert dietary cholesterol into the mature forms of the hormone 20-hydroxyecdysone.[2] For the most part these glandular tissues are lost in the adult with exception of the fat body, which is retained as a sheath of lipid tissue surrounding the brain and organs of the abdomen. In the adult female the ovary is a substantial source of 20-hydroxyecdysone production.[3] Adult males are left with, so far as is currently known, one source of 20-hydroxyecdysone which is the fat body tissue. These hormone producing tissues express the ecdysone receptor throughout development, possibility indicating a functional feedback mechanism.
Ecdysteroid activity in arthropods
An ecdysteroid is a type of steroid hormones in insects that are derived from enzymatic modification of cholesterol by p450 enzymes. This occurs by a mechanism similar to steroid synthesis in vertebrates. Ecdysone and 20-hydroxyecdysone regulate larval molts, onset of puparium formation, and metamorphosis. Being that these hormones are hydrophobic, they traverse lipid membranes and permeate the tissues of an organism. Indeed, the main receptor of these hormone signals - the ecdysone receptor - is an intracellular protein.
In humans and other mammals
Use as supplement
20-Hydroxyecdysone and other ecdysteroids are marketed as ingredients in nutritional supplements for various sports, particularly bodybuilding.[1] The evidence to support such use, however, is limited.[1] A comprehensive study, designed to find any strength or athletic improvement from 20-hydroxyecdysone, was published in 2006. The study looked for improvement in actual exercises performed and tested for improvements/increases in chemical indicators such as body composition and free/available testosterone. The study concluded that supplementation with up to 200 mg per day had no effect.[4]
Use as research tool
20-Hydroxyecdysone and other ecdysteroids are used in biochemistry research as inducers in transgenic animals, whereby a new gene is introduced into an animal so that its expression is under the control of an introduced ecdysone receptor. Adding or removing ecdysteroids from the animal's diet then gives a convenient way to turn the inserted gene on or off (see ecdysone receptor). At usual doses, 20-hydroxyecdysone appears to have little or no effect on animals that do not have extra genes inserted; it also has high bioavailability when taken orally, so it is useful for determining whether the transgene has been taken up effectively.[5] For uses in gene therapy, it may be necessary to investigate more thoroughly the natural sources of ecdysteroids in humans (which appear to include dietary phytoecdysteroids, gut flora, helminth infections, and other diseases).[6]
There is some in vitro evidence to show that 20-hydroxyecdysone has effects on some kinds of blood cells such as lymphocytes and neutrophils, and may act as an immunomodulator.[7] This would explain the mechanism behind Helminthic therapy due to the production of ecdysteroids by helminths, as mentioned above.
References
- 1 2 3 Lafont, R.; Dinan, L. (2003). "Practical uses for ecdysteroids in mammals including humans: an update" (PDF). J. Insect Sci. 3 (7).
- ↑ C. S. Thummel; J. Chory (2002). "Steroid signaling in plants and insects — common themes, different pathways". Genes & Development. 16 (24): 3113–3129. doi:10.1101/gad.1042102. PMID 12502734.
- ↑ A. M. Handler (1982). "Ecdysteroid titers during pupal and adult development in Drosophila melanogaster". Developmental Biology. 93 (1): 73–82. doi:10.1016/0012-1606(82)90240-8. PMID 6813165.
- ↑ Wilborn, Colin D; Taylor, Lemuel W; Campbell, Bill I; Kerksick, Chad; Rasmussen, Chris J; Greenwood, Michael; Kreider, Richard B (2006). "Effects of Methoxyisoflavone, Ecdysterone, and Sulfo-Polysaccharide Supplementation on Training Adaptations in Resistance-Trained Males". Journal of the International Society of Sports Nutrition. 3 (2): 19. doi:10.1186/1550-2783-3-2-19.
- ↑ E. Saez; M. C. Nelson; B. Eshelman; E. Banayo; A. Koder; G. J. Cho; R. M. Evans (2000). "Identification of ligands and coligands for the ecdysone-regulated gene switch" (PDF). Proceedings of the National Academy of Sciences. 97 (26): 14512–14517. doi:10.1073/pnas.260499497. PMC 18950
 . PMID 11114195.
. PMID 11114195. - ↑ Graham, Lloyd D (2002). "Ecdysone-controlled expression of transgenes". Expert Opinion on Biological Therapy. 2 (5): 525–35. doi:10.1517/14712598.2.5.525. PMID 12079488.
- ↑ D. S. Trenin; V. V. Volodin (1999). "20-hydroxyecdysone as a human lymphocyte and neutrophil modulator: in vitro evaluation". Archives of Insect Biochemistry and Physiology. 41 (3): 156–161. doi:10.1002/(SICI)1520-6327(1999)41:3<156::AID-ARCH7>3.0.CO;2-Q.