3,5-Di-tert-butylsalicylaldehyde
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
3,5-Di-tert-butyl-2-hydroxybenzaldehyde | |||
| Identifiers | |||
| 37942-07-7 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEMBL | ChEMBL427197 | ||
| ChemSpider | 599518 | ||
| ECHA InfoCard | 100.157.837 | ||
| PubChem | 688023 | ||
| |||
| |||
| Properties | |||
| C15H22O2 | |||
| Molar mass | 234.34 g·mol−1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
3,5'-Di-tert-butylsalicylaldehyde is an organic compound. It is a pale yellow solid. This aldehyde is a building block for preparing salen ligands.
Preparation
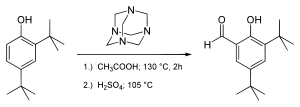
This compound is commercially available. It may be prepared by the Duff reaction, from 2,4-di-tert-butylphenol and hexamethylenetetramine:[1]
Reactions
Condensation of this aldehyde with various diamines gives various salen ligands, which are popular for catalysis. For example, the enantiopure ligand for Jacobsen's catalyst may be prepared from the appropriate 1,2-diaminocyclohexane. The catalyst is obtained by further reaction with manganese(II) acetate and atmospheric oxygen in the presence of a chloride source.[1]
References
- 1 2 Jay F. Larrow and Eric N. Jacobsen (2004). "(R,R)-N,N'-Bis(3,5-Di-tert-Butylsalicylidene)-1,2-Cyclohexanediamino Manganese(III) Chloride, A Highly Enantioselective Epoxidation Catalyst". Org. Synth.; Coll. Vol., 10, p. 96
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
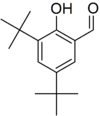

-Jacobsen's_catalyst.png)