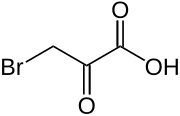
Bromopyruvic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Bromo-2-oxopropanoic acid | |
| Other names
Bromopyruvate 3-Bromopyruvic acid 3-Bromopyruvate 3-BrPA 3BP 3-Br-Pyr | |
| Identifiers | |
| 1113-59-3 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:131461 |
| ChEMBL | ChEMBL177837 |
| ChemSpider | 63850 |
| ECHA InfoCard | 100.012.915 |
| PubChem | 70684 |
| |
| |
| Properties | |
| C3H3BrO3 | |
| Molar mass | 166.96 g·mol−1 |
| Melting point | 79 to 82 °C (174 to 180 °F; 352 to 355 K) (hydrate) |
| Hazards | |
| R-phrases | R34 |
| S-phrases | S25 S36/37/39 S45 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Bromopyruvic acid and its alkaline form, bromopyruvate, are synthetic brominated derivatives of pyruvic acid. They are lactic acid and pyruvate analogs.
The characteristics of bromopyruvate in vitro and in vivo have been reported in the scientific literature since the 1940s. Because it is a highly reactive alkylating agent and it is inherently unstable, it had been described as a metabolic poison.
Research activity
In 1993, researchers at the Johns Hopkins School of Medicine reported that a pyruvate transporter system could be used to deliver bromopyruvate inside trypanosomal cells where its primary target is glyceraldehyde-3-phosphate dehydrogenase, which is highly sensitive to inhibition by bromopyruvate.[1] The pyruvate transporter system, which is known to be overexpressed in cancer cells, was later identified to be a monocarboxylate transporter called monocarboxylate transporter 1.[2]
References
- ↑ Barnard, JP; Reynafarje, B; Pedersen, PL (1993). "Glucose catabolism in African trypanosomes. Evidence that the terminal step is catalyzed by a pyruvate transporter capable of facilitating uptake of toxic analogs". The Journal of Biological Chemistry. 268 (5): 3654–3661. PMID 8429041.
- ↑ Liu, Zhe; Sun, Yiming; Hong, Haiyu; Zhao, Surong; Zou, Xue; Ma, Renqiang; Jiang, Chenchen; Wang, Zhiwei; Li, Huabin (2015-08-15). "3-bromopyruvate enhanced daunorubicin-induced cytotoxicity involved in monocarboxylate transporter 1 in breast cancer cells". American Journal of Cancer Research. 5 (9): 2673–2685. ISSN 2156-6976. PMC 4633897
 . PMID 26609475.
. PMID 26609475.
External links
- Mathupala, Saroj P.; Ko, Young H.; Pedersen, Peter L. (2010). "The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1797 (6–7): 1225–1230. doi:10.1016/j.bbabio.2010.03.025.
- Mathupala, Saroj P.; Ko, Young H.; Pedersen, Peter L. (2009). "Hexokinase-2 bound to mitochondria: Cancer's stygian link to the "Warburg effect" and a pivotal target for effective therapy". Seminars in Cancer Biology. 19 (1): 17–24. doi:10.1016/j.semcancer.2008.11.006. PMC 2714668
 . PMID 19101634.
. PMID 19101634. - Ko, Young H.; Smith, Barbara L.; Wang, Yuchuan; Pomper, Martin G.; Rini, David A.; Torbenson, Michael S.; Hullihen, Joanne; Pedersen, Peter L. (2004). "Advanced cancers: Eradication in all cases using 3-bromopyruvate therapy to deplete ATP". Biochemical and Biophysical Research Communications. 324 (1): 269–75. doi:10.1016/j.bbrc.2004.09.047. PMID 15465013.
- Mathupala, S P; Ko, Y H; Pedersen, P L (2006). "Hexokinase II: Cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria". Oncogene. 25 (34): 4777–86. doi:10.1038/sj.onc.1209603. PMC 3385868
 . PMID 16892090.
. PMID 16892090. - The cancer cell's "power plants" as promising therapeutic targets: An overview, by Peter Pederson