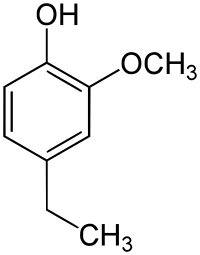
4-Ethylguaiacol
 | |
| Names | |
|---|---|
| IUPAC name
4-Ethyl-2-methoxy-phenol | |
| Other names
p-Ethylguaiacol Homocresol Guaiacyl ethane 2-Methoxy-4-ethylphenol | |
| Identifiers | |
| 2785-89-9 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 56245 |
| ECHA InfoCard | 100.018.637 |
| PubChem | 62465 |
| UNII | C9NFD83BJ5 |
| |
| |
| Properties | |
| C9H12O2 | |
| Molar mass | 152.19 g·mol−1 |
| Appearance | Colorless liquid |
| Melting point | 15 °C (59 °F; 288 K) |
| Boiling point | 234 to 236 °C (453 to 457 °F; 507 to 509 K) |
| Hazards | |
| Safety data sheet | External MSDS |
| EU classification (DSD) |
|
| S-phrases | S26 S37/39 |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
4-Ethylguaiacol, often abbreviated to 4-EG, is a phenolic compound with the molecular formula C9H12O2. It is produced along with 4-ethylphenol (4-EP) in wine and beer by the spoilage yeast Brettanomyces.[1] When it is produced by the yeast to concentrations greater than the sensory threshold of >600 µg/L, it can contribute bacon, spice, clove, or smoky aromas to the wine. On their own these characters can be quite attractive in a wine, however as the compound usually occurs with 4-EP whose aromas can be more aggressive, the presence of the compound often signifies a wine fault. The ratio in which 4-EP and 4-EG are present can greatly affect the organoleptic properties of the wine.
See also
References
- ↑ Caboni, Pierluigi; Sarais, Giorgia; Cabras, Marco; Angioni, Alberto (2007). "Determination of 4-Ethylphenol and 4-Ethylguaiacol in Wines by LC-MS-MS and HPLC-DAD-Fluorescence". Journal of Agricultural and Food Chemistry. 55 (18): 7288–93. doi:10.1021/jf071156m. PMID 17676867.
This article is issued from Wikipedia - version of the 10/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
