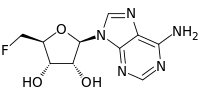
5'-Deoxy-5'-fluoroadenosine
 | |
| Names | |
|---|---|
| IUPAC name
5’-Deoxy-5’-fluoroadenosine | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 395211 |
| PubChem | 448403 |
| |
| |
| Properties | |
| C10H12FN5O3 | |
| Molar mass | 269.24 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5'-Deoxy-5'-fluoroadenosine is the first step in the biosynthesis of organic fluorides. It is synthesized by the fluorinase catalyzed addition of a fluoride ion to S-adenosyl-L-methionine, releasing L-methionine as a by product.[1] Purine nucleoside phosphorylase mediates a phosphorolytic cleavage of the adenine base to generate 5-fluoro-5-deoxy-D-ribose-1-phosphate.
References
- ↑ O'Hagan D, Schaffrath C, Cobb SL, Hamilton JT, Murphy CD (2002). "Biochemistry: biosynthesis of an organofluorine molecule". Nature. 416 (6878): 279. doi:10.1038/416279a. PMID 11907567.
This article is issued from Wikipedia - version of the 5/18/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.