Irofulven
 | |
| Names | |
|---|---|
| IUPAC name
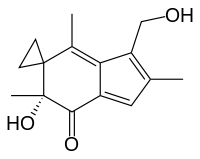
(6'R)-6'-hydroxy-3'-(hydroxymethyl)-2',4',6'-trimethylspiro[cyclopropane-1,5'-inden]-7'(6'H)-one | |
| Identifiers | |
| 158440-71-2 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL118218 |
| ChemSpider | 130640 |
| KEGG | D04614 |
| PubChem | 148189 |
| UNII | 6B799IH05A |
| |
| |
| Properties | |
| C15H18O3 | |
| Molar mass | 246.302 g/mol |
| Density | 1.285 g/mL |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Irofulven or 6-hydroxymethylacylfulvene (also known as HMAF of MGI-114) is an experimental antitumor agent.[2][3] It belongs to the family of drugs called alkylating agents.
It inhibits the replication of DNA in cell culture.[4]
Irofulven is an analogue of illudin S, a sesquiterpene toxin found in the Jack 'o' Lantern mushroom (Omphalotus illudens). The compound was originally synthesized by Dr. Trevor McMorris and found to have anticancer properties in mice by Dr. Michael J Kelner.[5]
Synthesis
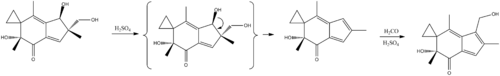
McMorris, T. C.; Staake, M. D.; Kelner, M. J. (2004). "Synthesis and Biological Activity of Enantiomers of Antitumor Irofulven". The Journal of Organic Chemistry. 69 (3): 619–23. doi:10.1021/jo035084j. PMID 14750783.
References
- ↑ Aktietorget
- ↑ Escargueil, A. E.; Poindessous, V.; Soares, D. G.; Sarasin, A.; Cook, P. R.; Larsen, A. K. (April 2008). "Influence of Irofulven, a Transcription-Coupled Repair-Specific Antitumor Agent, on RNA Polymerase Activity, Stability and Dynamics in Living Mammalian Cells" (pdf). Journal of Cell Science. 121 (Pt 8): 1275–1283. doi:10.1242/jcs.023259. PMID 18388315.
- ↑ Kelner, M. J.; McMorris, T. C.; Estes, L.; Wang, W.; Samson, K. M.; Taetle, R. (1996). "Efficacy of HMAF (MGI-114) in the MV522 Metastatic Lung Carcinoma Xenograft Model Nonresponsive to Traditional Anticancer Agents". Investigational New Drugs. 14 (2): 161–167. doi:10.1007/BF00210787. PMID 8913837.
- ↑ Wang, Y.; Wiltshire, T.; Senft, J.; Reed, E.; Wang, W. (February 2007). "Irofulven Induces Replication-Dependent CHK2 Activation Related to p53 Status" (pdf). Biochemical Pharmacology. 73 (4): 469–480. doi:10.1016/j.bcp.2006.10.023. PMC 1800887
 . PMID 17118344.
. PMID 17118344. - ↑ MacDonald, J. R.; Muscoplat, C. C.; Dexter, D. L.; Mangold, G. L.; Chen, S. F.; Kelner, M. J.; McMorris, T. C.; Von Hoff, D. D. (1997). "Preclinical Antitumor Activity of 6-hydroxymethylacylfulvene, a Semisynthetic Derivative of the Mushroom Toxin Illudin S" (pdf). Cancer Research. 57 (2): 279–283. PMID 9000568.
This article is issued from Wikipedia - version of the 6/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.