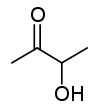
Acetoin
 | |
| Names | |
|---|---|
| Other names
3-Hydroxybutanone Acetyl methyl carbinol | |
| Identifiers | |
| 513-86-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15688 |
| ChemSpider | 21105851 |
| ECHA InfoCard | 100.007.432 |
| EC Number | 208-174-1 |
| KEGG | C01769 |
| PubChem | 179 |
| RTECS number | EL8790000 |
| UNII | BG4D34CO2H |
| |
| |
| Properties | |
| C4H8O2 | |
| Molar mass | 88.11 g·mol−1 |
| Appearance | slightly yellow liquid or crystals |
| Odor | bland, yogurt-like |
| Density | 1.012 g/cm3 |
| Melting point | 15 °C (59 °F; 288 K) |
| Boiling point | 148 °C (298 °F; 421 K) |
| Soluble | |
| Solubility in other solvents | Soluble in alcohol Slightly soluble in ether, petroleum ether Miscible in propylene glycol Insoluble in vegetable oil |
| log P | -0.36 |
| Acidity (pKa) | 13.72 |
| Chiral rotation ([α]D) |
-39.4 |
| Refractive index (nD) |
1.4171 |
| Hazards | |
| Safety data sheet | MSDS |
| Flash point | 41 °C (106 °F; 314 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
> 5000 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Acetoin, also known as 3-hydroxybutanone or acetyl methyl carbinol, with the molecular formula is C4H8O2, is a colorless or pale yellow to green yellow liquid with a pleasant, buttery odor. Acetoin is a chiral molecule. The form produced by bacteria is (R)-acetoin.[1]
Production in bacteria
Acetoin is a neutral, four-carbon molecule used as an external energy store by a number of fermentive bacteria. It is produced by the decarboxylation of alpha-acetolactate, a common precursor in the biosynthesis of branched-chain amino acids. Owing to its neutral nature, production and excretion of acetoin during exponential growth prevents overacidification of the cytoplasm and the surrounding medium that would result from accumulation of acidic metabolic products, such as acetic acid and citric acid. Once superior carbon sources are exhausted, and the culture enters stationary phase, acetoin can be used to maintain the culture density.[2] The conversion of acetoin into acetyl-CoA is catalysed by the acetoin dehydrogenase complex, following a mechanism largely analogous to the pyruvate dehydrogenase complex; however, as acetoin is not a 2-oxoacid, it does not undergo decarboxylation by the E1 enzyme; instead, a molecule of acetaldehyde is released.[3] In some bacteria, acetoin can also be reduced to 2,3-butanediol by acetoin reductase/2,3-butanediol dehydrogenase.
The Voges-Proskauer test is a commonly used microbiological test for acetoin production.[4]
In food products
Acetoin, along with diacetyl, is one of the compounds giving butter its characteristic flavor. Because of this, manufacturers of partially hydrogenated oils typically add artificial butter flavor - acetoin and diacetyl - (along with beta carotene for the yellow color) to the final product, which would otherwise be tasteless.[5]
Acetoin is used as a food flavoring (in baked goods) and as a fragrance. It can be found in apples, butter, yogurt, asparagus, blackcurrants, blackberries, wheat, broccoli, brussels sprouts, cantaloupes and maple syrup.
Cigarette additive
In a 1994 report released by five top cigarette companies, acetoin was listed as one of the 599 additives to cigarettes. [6]
References
- ↑ Albert Gossauer: Struktur und Reaktivität der Biomoleküle, Verlag Helvetica Chimica Acta, Zürich, 2006, Seite 285, ISBN 978-3-906390-29-1.
- ↑ Xiao, Z.; Xu, P. (2007). "Acetoin metabolism in bacteria". Crit Rev Microbiol. 33 (2): 127–140. doi:10.1080/10408410701364604. PMID 17558661.
- ↑ Oppermann, F.B.; Steinbuchel, A. (1994). "Identification and molecular characterization of the aco genes encoding the Pelobacter carbinolicus acetoin dehydrogenase enzyme system". J Bacteriol. 176 (2): 469–485. PMC 205071
 . PMID 8110297.
. PMID 8110297. - ↑ Speckman, R.A.; Collins, E.B. (1982). "Specificity of the Westerfeld adaptation of the Voges-Proskauer test". Appl Environ Microbiol. 44 (1): 40–43. PMC 241965
 . PMID 6751225.
. PMID 6751225. - ↑ Pavia et al., Introduction to Organic Laboratory Techniques, 4th ed., ISBN 978-0-495-28069-9
- ↑ "What's in a cigarette?". Archived from the original on 23 May 2006. Retrieved 2006-05-31.