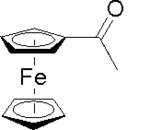
Acetylferrocene
 | |
| Names | |
|---|---|
| Other names
Acetylferrocene | |
| Identifiers | |
| 1271-55-2 | |
| ECHA InfoCard | 100.013.676 |
| Properties | |
| [Fe(C5H4COCH3)(C5H5)] | |
| Molar mass | 228.07 g/mol |
| Appearance | Red brown crystal |
| Density | 1.014 g/mL |
| Melting point | 81 to 83 °C (178 to 181 °F; 354 to 356 K) [1] |
| Boiling point | 161 to 163 °C (322 to 325 °F; 434 to 436 K) (4 mmHg) |
| Insoluble in water, soluble in most organic solvents | |
| Hazards | |
| EU classification (DSD) |
|
| R-phrases | R24, R28 |
| S-phrases | (S1), S28, S36/37/39, S48[2] |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
25 mg kg−1 (oral, rat) 50 mg kg−1 (oral, mouse)[3] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |

Acetylferrocene is the organoiron compound with the formula (C5H5)Fe(C5H4COMe). It consists of ferrocene substituted by an acetyl group on one of the cyclopentadienyl rings. It is an orange, air-stable solid that is soluble in organic solvents.
Preparation and reactions
Acetylferrocene is prepared by Friedel-Crafts acylation of ferrocene, usually with acetic anhydride. The complete preparation from aromatization of dicyclopentadiene to ferrocene is:
The experiment is often conducted in the instructional laboratory to illustrate acylation as well as chromatographic separations.[4][5]
Acetylferrocene can be converted to many derivatives, e.g., reduction to the chiral alcohol (C5H5)Fe(C5H4CH(OH)Me) and precursor to vinylferrocene. The oxidized derivative, acetylferrocenium, is used as a 1e-oxidant in the research laboratory.[6]
References
- ↑ Sigma-Aldrich Co., Acetylferrocene. Retrieved on 2013-07-20.
- ↑ https://fscimage.fishersci.com/msds/69220.htm
- ↑ http://msds.chem.ox.ac.uk/AC/acetylferrocene.html
- ↑ Bozak, R. E. "Acetylation of ferrocene: A chromatography experiment for elementary organic laboratory" J. Chem. Educ., 1966, volume 43, p 73.doi:10.1021/ed043p73
- ↑ Donahue, C. J., Donahue, E. R., "Beyond Acetylferrocene: The Synthesis and NMR Spectra of a Series of Alkanoylferrocene Derivatives", Journal of Chemical Education 2013, volume 90, pp. 1688. doi:10.1021/ed300544n
- ↑ Connelly, N. G., Geiger, W. E., "Chemical Redox Agents for Organometallic Chemistry", Chem. Rev. 1996, 96, 877. doi:10.1021/cr940053x

