Adelmidrol
 | |
| Names | |
|---|---|
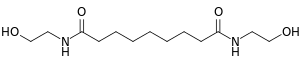
| IUPAC name
N,N′-Bis(2-hydroxyethyl)nonanediamide | |
| Identifiers | |
| 1675-66-7 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL2105967 |
| ChemSpider | 154047 |
| PubChem | 176874 |
| |
| |
| Properties | |
| C13H26N2O4 | |
| Molar mass | 274.36 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Adelmidrol is an anti-inflammatory ethanolamide derivative of azelaic acid.[1]
Description
Adelmidrol is the diethanolamide derivative of azelaic acid, i.e., naturally occurring dicarboxylic acid that has long proven to be an effective topical treatment for human inflammatory skin disorders,[2] and whose mechanism of action have been recently and thoroughly investigated.[3]
Similarly to the anti-inflammatory and anti-nociceptive compound palmitoylethanolamide (PEA),[4][5][6][7][8][9][10][11][12][13][14][15][16][17][18] adelmidrol belongs to the aliamide family, a group of fatty acid derivatives with cannabimimetic properties, able to control the event of mast cell (MC) hyper-reactivity in several pathophysiological and pathological conditions.[6]
Adelmidrol is a synthetic compound with a symmetrical chemical structure. It is used extensively in Italy in veterinary medicine, to treat skin inflammation. In 2015 it was found the compound also exerts anti-inflammatory action given systemically in 10 mg per kg bodyweight.[2]
Adelmidrol in pet animals and in atopic eczema
Adelmidrol has been shown to negatively control the behavior of canine skin MCs during pathophysiological conditions (i.e. healing of experimental wounds). In particular, a statistically significant increase of intracytoplasmatic granular content of dermal MCs was shown in adelmidrol (2%)-treated wounds compared to control, thus suggesting the compound is effectively able to down-modulate skin MC degranulation in dogs.[19]
Chronic gingiva inflammation can be a difficult to treat medical problem in dogs. A similar anti-inflammatory effect was observed in these dogs, treated with a gel to reduce gingival inflammation. Twenty dogs were randomised to the adelmidrol gel and placebo. After 30 and 45 days, the dogs using the adelmidrol gel had significantly less inflammation of the gingiva.
Furthermore, the local application of adelmidrol has been recently confirmed to reduce MC responses during chronic experimental inflammation, as shown by the significant decrease of mediators selectively expressed by MCs and involved in skin inflammation, such as chymase.[20]
Adelmidrol seems well suitable for topical application, because exhibits both hydrophilic and lipophilic features, which help to absorb more effectively into the skin, epidermis being composed of alternating lipophilic and hydrophilic layers.
A 4-week topical treatment with adelmidrol 2% emulsion in children affected by mild atopic dermatitis resulted in complete resolution in 80% of cases, with no side effects and no relapses at 8-week follow up.[21]
References
- ↑ De Filippis, D; d'Amico, A; Cinelli, MP; Esposito, G; Di Marzo, V; Iuvone, T (2009). "Adelmidrol, a palmitoylethanolamide analogue, reduces chronic inflammation in a carrageenin-granuloma model in rats". Journal of cellular and molecular medicine. 13 (6): 1086–95. doi:10.1111/j.1582-4934.2008.00353.x. PMID 18429935.
- 1 2 Nazzaro-Porro M: Azelaic acid. J Am Acad Dermatol 1987, 17:1033-1041.
- ↑ Mastrofrancesco A, Ottaviani M, Aspite N, Cardinali G, Izzo E, Graupe K, Zouboulis CC, Camera E, Picardo M: Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARgamma activation. Exp Dermatol 2010, 19:813-820.
- ↑ Costa B, Comelli F, Bettoni I, Colleoni MP, Giagnoni G: The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB1, TRPV1 and PPARgamma receptors and neurotrophic factors. Pain 2008, 139:541-550.
- ↑ Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 2005, 67:15-19.
- 1 2 Re G, Barbero R, Miolo A, Di Marzo V: Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: Potential use in companion animals. Vet J 2007, 173:23-32.
- ↑ Wise LE, Cannavacciuolo R, Cravatt BF, Marun BF, Lichtman AH: Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology 2008, 54:181-188.
- ↑ Genovese T, Esposito E, Mazzon E, Di Paola R, Meli R, Bramanti P, Piomeli D, Calignano A, Cuzzocrea S: Effects of palmitoylethanolamide on signaling pathways implicated in the development of spinal cord injury. J Pharmacol Exp Ther 2008, 326:12-23.
- ↑ De Filippis D, Luongo L, Cipriano M, Palazzo E, Cinelli MP, de Novellis V, Maione S, Iuvone T: Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol Pain 2011, 7:3.
- ↑ Luongo L, Guida F, Gatta L, de Novellis V, Maione S: Palmitoylethanolamide systemic treatment reduces spinal and supraspinal formalin-induced neuroinflammation and allodynia. Shock 2011, 36(suppl1):22.
- ↑ Sasso O, Russo R, Vitiello S, Mattace Raso G, D’Agostino G, Iacono A, La Rana G, Vallée M, Cuzzocrea S, Piazza PV, Meli R, Calignano A: Implication of allopregnanolone in the antinociceptive effect of N-palmitoylethanolamide in acute or persistent pain. Pain 2012, 153:33-41.
- ↑ Truini A, Biasiotta A, Di Stefano G, Cesa SL, Leone C, Cartoni C, Federico V, Petrucci MT, Cruccu G: Palmitoylethanolamide Restores Myelinated-Fibre Function in Patients with Chemotherapy-Induced Painful Neuropathy. CNS Neurol Disord Drug Targets 2011, 10:916-920.
- ↑ Keppel Hesselink JM: New Targets in pain, non-neuronal cells, and the role of palmitoylethanolamide. Mini-Review. The Open Pain Journal 2012, 5:12-23.
- ↑ Aloe, L, Leon, A, Levi-Montalcini, R: A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions 1993, 39(Spec No):C145–C147.
- ↑ Jack DB: Aliamides: a new approach to the treatment of inflammation. Drug News Perspect 1996, 9:93–98.
- ↑ De Filippis D, D’Amico A, Iuvone T: Cannabinomimetic control of mast cell mediator release: new perspective in chronic inflammation. J Neuroendrocrinol 2008, 20 (Suppl 1):20–25.
- ↑ Cantarella G, Scollo M, Lempereur L, Saccani-Jotti G, Basile F, Bernardini R: Endocannabinoids inhibit release of nerve growth factor by inflammation-activated mast cells. Biochem Pharmacol 2011, 82:380-388.
- ↑ Esposito E, Paterniti I, Mazzon E, Genovese T, Di Paola R, Galuppo M, Cuzzocrea S: Effects of palmitoylethanolamide on release of mast cell peptidases and neurotrophic factors after spinal cord injury. Brain Behav Immun 2011, 25:1099-1112.
- ↑ Abramo F, Salluzzi D, Leotta R, Auxilia S, Noli C, Miolo A, Mantis P, Lloyd DH: Mast Cell Morphometry and Densitometry in Experimental Skin Wounds Treated With a Gel Containing Adelmidrol: A Placebo Controlled Study. Wounds 2008, 20:149-157.
- ↑ De Filippis D, D’Amico A, Cinelli MP, Esposito G, Di Marzo V, Iuvone T: Adelmidrol, a palmitoyethanolamide analogue, reduces chronic inflammation in a carrageenin-granuloma model in rats. J Cell Mol Med 2009, 13:1086-1095
- ↑ Pulvirenti N, Nasca MR, Micali G: Topical adelmidrol 2% emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: a pilot study. Acta Dermatovenerol Croat 2007, 15:80-83.
-
 This article incorporates text by Santiago Cerrato, Pilar Brazis, Maria Federica della Valle, Alda Miolo and Anna Puigdemont available under the CC BY 2.0 license.
This article incorporates text by Santiago Cerrato, Pilar Brazis, Maria Federica della Valle, Alda Miolo and Anna Puigdemont available under the CC BY 2.0 license.