Alcohol oxidation
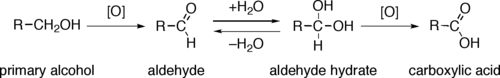
Alcohol oxidation is an important organic reaction. Primary alcohols (R-CH2-OH) can be oxidized either to aldehydes (R-CHO) or to carboxylic acids (R-CO2H), while the oxidation of secondary alcohols (R1R2CH-OH) normally terminates at the ketone (R1R2C=O) stage. Tertiary alcohols (R1R2R3C-OH) are resistant to oxidation.[1]
The direct oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (R-CH(OH)2) by reaction with water before it can be further oxidized to the carboxylic acid.
Often it is possible to interrupt the oxidation of a primary alcohol at the aldehyde level by performing the reaction in absence of water, so that no aldehyde hydrate can be formed.
Oxidation to aldehydes
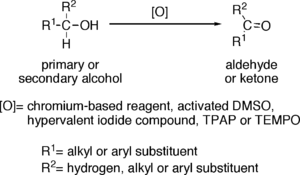
Oxidation of alcohols to aldehydes is partial oxidation; aldehydes are further oxidized to carboxylic acids. Conditions required for making aldehydes are heat and distillation. In aldehyde formation, the temperature of the reaction should be kept above the boiling point of the aldehyde and below the boiling point of the alcohol.
Reagents useful for the transformation of primary alcohols to aldehydes are normally also suitable for the oxidation of secondary alcohols to ketones. These include:
- Chromium-based reagents, such as Collins reagent (CrO3·Py2), PDC or PCC.
- Activated DMSO, resulting from reaction of DMSO with electrophiles, such as oxalyl chloride (Swern oxidation), a carbodiimide (Pfitzner-Moffatt oxidation) or the complex SO3·Py (Parikh-Doering oxidation).
- Hypervalent iodine compounds, such as Dess-Martin periodinane or 2-Iodoxybenzoic acid.
- Catalytic TPAP in presence of excess of NMO (Ley oxidation).
- Catalytic TEMPO in presence of excess bleach (NaOCl) (Oxoammonium-catalyzed oxidation).
Allylic and benzylic alcohols can be oxidized in presence of other alcohols using certain selective oxidants such as manganese dioxide (MnO2).
Oxidation to ketones
Reagents useful for the oxidation of secondary alcohols to ketones, but normally inefficient for oxidation of primary alcohols to aldehydes, include chromium trioxide (CrO3) in a mixture of sulfuric acid and acetone (Jones oxidation) and certain ketones, such as cyclohexanone, in the presence of aluminium isopropoxide (Oppenauer oxidation). Another method is oxoammonium-catalyzed oxidation.
Oxidation to carboxylic acids

The direct oxidation of primary alcohols to carboxylic acids can be carried out using
- Potassium permanganate (KMnO4);
- Jones oxidation;
- PDC in DMF;
- Heyns oxidation;
- Ruthenium tetroxide (RuO4);
- or TEMPO.
Diol oxidation
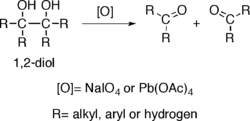
Alcohols possessing two hydroxy groups located on adjacent carbons —that is, 1,2-diols— suffer oxidative breakage at a carbon-carbon bond with some oxidants such as sodium periodate (NaIO4) or lead tetraacetate (Pb(OAc)4), resulting in generation of two carbonyl groups. The reaction is also known as glycol cleavage.
References
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7