Aluminized steel

Aluminized steel may be steel that might have been hot-dip coated on both sides with aluminium-silicon alloy. This process might assure a tight metallurgical bond between the steel sheet and its aluminium coating,possibly producing a material with a unique combination of properties possessed neither by steel nor by aluminium alone. Aluminized steel could show a better behavior against corrosion[1] and keeps the properties of the base material steel for temperature lower than 800 °C (1,470 °F). For example, it would be commonly used for heat exchangers in residential furnaces, commercial rooftop HVAC units, automotive mufflers, ovens, kitchen ranges, water heaters, fireplaces, barbecue burners, and baking pans. This steel could be very useful for heating things up because it transfers heat faster than most other steels.
Characteristics are defined by the exact metals and processes used.
Types
- Type 1
- Hot-dip coated with a thin layer of aluminium / silicon alloy containing 5% to 11% silicon to promote better adherence. It is intended principally for heat resisting applications and also for uses where corrosion resistance and heat are involved. Possible end uses are mufflers, furnaces, ovens, ranges, heaters, water heaters, fireplaces, and baking pans. Aluminized steel can withstand 550 °C (1,022 °F) with almost no change in the base material. But due to silicon content it develops black spot. Aluminized steel has slowly started to convert bakery trays which were previously made by galvanized or galvalume steel as it does not contain lead which is poisonous. Type 1 is also commonly found in industrial products.
- Type 2
- Hot-dip coated with commercially pure aluminum. It is intended principally for applications requiring atmospheric corrosion resistance. Type 2 may ultimately be manufactured into corrugated roofing and siding, grain bins, drying ovens, and air-conditioner condenser housings.
Properties
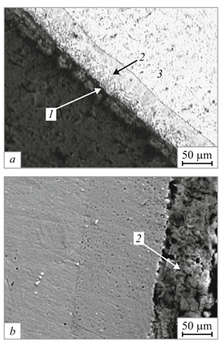
The basic structure of aluminized steel is a thin aluminium oxide layer outside, then an intermetallic layer that is a mix of aluminium, silicon, and steel, and finally a steel core.[2]
Both Type 1 and Type 2 show excellent high reflectivity characteristics. At temperatures up to 842 °C (1,548 °F), aluminized steel reflects up to 80% of heat projected onto it.[3] Aluminized steel has the ability to maintain its strength at temperatures up to 677 °C (1,251 °F). Although stainless steel is the stronger of the two, aluminized steel has a greater electrostatic surface, and can therefore reflect heat better.
Aluminized steel is highly resistant to corrosion because of the thin layers of aluminium and silicon, which keep the underlying steel from oxidizing. These thin layers also keep pit corrosion from occurring, especially during exposure to salts that affect most other metals. However, despite the good corrosion resistance of aluminized steel, if the aluminium layer is disrupted and the steel is exposed, then the steel may oxidize and corrosion may occur.
Consumption
In North America nearly 700,000 tons of aluminized steel are consumed annually.[4] Some of the common products made from aluminized steel include water heaters, ranges, furnaces, space heaters and grills.[5]
Processing
Aluminized steel can be made using a variety of processes, cladding, hot dipping, galvanic coating, metallizing, and calorizing, but the most effective process is hot dipping. The process of hot dipping starts by cleaning the steel, then placing the steel in a bath of Al-11%Si at a temperature of 988K and shaken, then pulled out and air dried.[6] The aluminium diffuses into the steel, creating an intermetallic layer above the steel base layer, but below the outside aluminum coating. The aluminium coating is oxidized to help protect the inner steel from corrosion and further aluminium diffusion.[7] The silicon is added to the aluminium bath to create a thinner layer of aluminium on the steel. The hot dipping process is cheaper and more effective to produce aluminized steel than any other process.[8]
GALVALUME
Galvalume was invented by Bethlehem Steel in 1972. It is a trademarked name, but many people use it as a generic term to describe a metal roofing product consisting of steel coil coated with a metal alloy. That alloy is 45% zinc and 55% aluminum and looks similar to galvanized steel, but the visible crystals are smaller and close together, giving it a smoother appearance. Galvalume has a cousin, Galvalume Plus. The only difference is Plus has a thin, clear acrylic coating. Because Galvalume Plus can be roll-formed dry without vanishing oil, it is very easy to form and install safely.
The combination of zinc and aluminum in Galvalume enhances both the positive and negative effects of aluminum. Galvalume has barrier corrosion resistance and heat resistance similar to aluminized material and good bare edge galvanic protection and forming qualities like galvanized material. Consequently, Galvalume and Galvalume Plus will resist rust, the elements and fire while providing a sturdy and protective covering.
Galvalume should not be used on, in, or around concrete or mortar. Concrete and mortar are highly alkaline environments. Bare Galvalume and painted Galvalume sheets suffer rapid corrosion when in contact with mortar and concrete. Bare Galvanized and painted Galvanized perform better in this type of environment.
GALVALUME Steel Sheet is carbon steel sheet coated with aluminium-zinc alloy by a continuous hot-dip process. The nominal coating composition is 55% aluminium and 45% zinc. A small but important addition of silicon is included in the coating alloy. It is added not to enhance the corrosion performance, but to provide good coating adhesion to the steel substrate when the product is roll-formed, drawn, or bent during fabrication. GALVALUME steel sheet combines the excellent barrier corrosion protection of aluminium with the galvanic protection of zinc. The result is a coating that lasts a long time, a coating that provides cut-edge protection along sheared edges, and therefore, a coating that offers excellent protection to steel sheet. Although there are a few exceptions, for most applications in most types of environment, GALVALUME steel sheet is the preferred product when long-term resistance to atmospheric corrosion is desired. It outlasts a galvanized coating of comparable thickness, and offers cut-edge protection that is not available with aluminium-coated sheet. This cut-edge protection means there is less rust-staining along sheared edges, at scratches, and other imperfections in the coating. Also, since the coating is so resistant to corrosion, it retains a very bright surface appearance when exposed to most atmospheres. These attributes make GALVALUME steel sheet the preferred material for roofing.
The superior corrosion resistance of GALVALUME steel sheet is achieved by the presence of microscopic zinc-rich and aluminium-rich areas within the coating. The aluminium-rich areas, which corrode very slowly, provide the long-term durability while the zinc-rich areas, which corrode preferentially, provide galvanic protection.
Galvalume metals are made with steel panels having a coating of corrosion resistant aluminium-zinc alloy applied by a continuous hot dip process. The nominal composition of the coating is 55% aluminium, 43.5% zinc and 1.5% silicon. Galvalume is the trade name for this patented sheet steel product. The alloy coating of aluminium and zinc combines the best properties of both metals. It has the corrosion resistance and heat reflectivity characteristic of aluminium coatings, with the formability and galvanic protection of cut edges characteristic of zinc coatings.
Galvalume is an excellent product for long-life roofing and siding material. The aluminum additive, along with the zinc, allows small particle areas to form within this coating. Any corrosion that occurs does so within these open particle areas. Galvanized metal corrodes in a linear fashion and eventually depletes the zinc coating entirely. Galvalume, on the other hand experiences some corrosion, but on a smaller and less significant scale. Galvalume will not breakdown completely, as galvanized will.
Uses

Aluminized steel was developed for providing more structural durability and a high yield strength in highly corrosive environments. Aluminized steel maintains the strength of high-alloy steel, but at a fraction of the cost. Aluminized steel is cheaper to produce than high-alloy steels and thus is a preferred material for manufacturing automobile and motorcycle exhaust gas systems.[8]
See also
References
- ↑ "Aluminized steel might offer Attractive Physical Characteristics For Use In Industrial Duct Construction". Sheet Metal and Air Conditioning Contractors' National Association. Retrieved 26 Feb 2011.
- ↑ Kee-Hyun, Kim. Van-Daele, Benny. Van-Tendeloo, Gusfaaf. and Jong-Kyu, Yoon. (2006). "Observations of Intermetallic Compound Formation of Hot Dip Aluminized steel". Materials Science Forum, 519-21(2), 1871-75.
- ↑ http://www.atlassteel.com/Products/aluminizedSteel.aspx
- ↑ http://www.blocksteel.com/facts.htm
- ↑ http://www.differencebetween.net/science/nature/difference-between-aluminized-steel-and-stainless-steel-2/
- ↑ Rajendran, R. Venkataswamy, S. Jaikrishna, U. Gowrishankar, N. and Rajadurai, A. (2006). "Effect of Process Parameters in Hot Dip Aluminizing of Medium Carbon Steel".
- ↑ Deqing, Wang. and Ziyuan, Shi. (2003) "Formation of Al2O3 Layer on Steel". Journal of Materials Science Letters, 22(14), 1003-1006.
- 1 2 Wang, Chaur. Jeng. Badaruddin, Mohd.. (2010) "The dependence of high temperature resistance of aluminized steel exposed to water-vapour oxidation". Surface and Coatings Technology, 205(5), 1200-1205.