Ammonium hexachloroiridate
 | |
2PtCl6Xray.tif.png) | |
| Identifiers | |
|---|---|
| 16940-92-4 | |
| ECHA InfoCard | 100.037.264 |
| Properties | |
| H8N2Cl6Ir | |
| Molar mass | 441.01 |
| Appearance | brown crystals |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
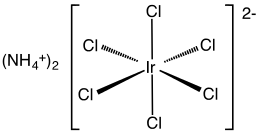
Ammonium hexachloroiridate is the inorganic compound with the formula (NH4)2[IrCl6]. This dark brown solid is the ammonium salt of the iridium(IV) complex [IrCl6]2-. It is a commercially important iridium compound.[1] It is one of the most common complexes of iridium(IV).
Structure
The compound has been characterized by X-ray crystallography. The salt crystallizes in a cubic motif like that of ammonium hexachloroplatinate. The [IrCl6]2- centers adopt octahedral molecular geometry.[2]
Uses
It is a key intermediate in the isolation of iridium from ores. Most other metals form insoluble sulfides when aqueous solutions of their chlorides are treated with hydrogen sulfide, but [IrCl6]2- resists ligand substitution. Upon heating under hydrogen, the solid salt converts to the metal:[1]
- (NH4)2[IrCl6] + 2 H2 → Ir + 6 HCl + 2 NH3
References
- 1 2 Renner, H.; Schlamp, G.; Kleinwächter, I.; Drost, E.; Lüschow, H. M.; Tews, P.; Panster, P.; Diehl, M.; et al. (2002). "Platinum group metals and compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a21_075.
- ↑ Bokii, G.B.; Ussikov, P.I. "Roentgenographische Untersuchung der Struktur des Ammonium-Chlor-Iridats (N H4)2IrCl6 Doklady Akademii Nauk SSSR 1940, vol. 26, p782-p784.