Amyrin
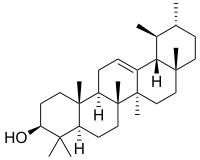 α-Amyrin | |
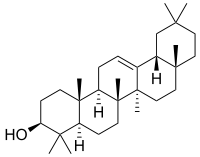 β-Amyrin | |
| Names | |
|---|---|
| IUPAC names
α: (3β)-Urs-12-en-3-ol β: (3β)-Olean-12-en-3-ol δ: (3β)-Olean-13(18)-en-3-ol | |
| Other names
α: α-Amyrenol; α-Amirin; α-Amyrine; Urs-12-en-3β-ol; Viminalol β: β-Amyrenol; β-Amirin; β-Amyrine; Olean-12-en-3β-ol; 3β-Hydroxyolean-12-ene | |
| Identifiers | |
| 638-95-9 (α) 559-70-6 (β) 508-04-3 (δ) | |
| 3D model (Jmol) | (α): Interactive image (β): Interactive image |
| ChemSpider | 65935 (α) 65921 (β) 26333109 (δ) |
| ECHA InfoCard | 100.010.321 |
| PubChem | 73170 (α) 73145 (β) 12358447 (δ) |
| |
| |
| Properties | |
| C30H50O | |
| Molar mass | 426.73 g·mol−1 |
| Melting point | α: 186 °C[1] β: 197-187.5 °C[2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The amyrins are three closely related natural chemical compounds of the triterpene class. They are designated α-amyrin, β-amyrin and δ-amyrin. Each is a pentacyclic triterpenol with the chemical formula C30H50O. They are widely distributed in nature and have been isolated from a variety of plant sources such as epicuticular wax. All three amyrins occur in the surface wax of tomato fruit.[3] α-Amyrin is found in dandelion coffee.
References
- ↑ Merck Index, 11th Edition, 653
- ↑ Merck Index, 11th Edition, 654
- ↑ Bauer, Stefan; Schulte, Erhard; Thier, Hans-Peter (2004). "Composition of the surface wax from tomatoes II. Quantification of the components at the ripe red stage and during ripening". European Food Research and Technology. 219: 487–491. doi:10.1007/s00217-004-0944-z.
This article is issued from Wikipedia - version of the 11/16/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.