Cyclen
 | |
| Names | |
|---|---|
| IUPAC name
1,4,7,10-tetrazacyclododecane | |
| Identifiers | |
| 294-90-6 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:37391 |
| ChEMBL | ChEMBL19880 |
| ChemSpider | 58488 |
| ECHA InfoCard | 100.102.391 |
| PubChem | 64963 |
| |
| |
| Properties | |
| C8H20N4 | |
| Molar mass | 172.271 |
| Appearance | White Solid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Cyclen or 1,4,7,10-tetraazacyclododecane is a macrocycle and the aza analogue of the crown ether 12-crown-4. Derivatives of cyclen are larger cyclic polyamines but the repeating unit (ethyleneimine, –CH2CH2NH–) is always the same. Like crown ethers, cyclen compounds are capable of selectively binding cations. They are used as a ligand in chemistry for instance with chemicals used in MRI contrast agents.
Synthesis
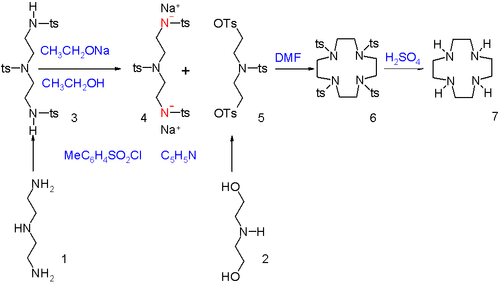
Cyclen compounds can be synthesized by combining two separate parts by nucleophilic displacement.[2] In this procedure the terminal amine groups in diethylene triamine (1) are activated as amine anionic nucleophiles by reaction with tosyl chloride in pyridine to the N-tosyl protective group followed by proton abstraction with sodium ethoxide. The alcohol end groups in diethanolamine (2) are activated as electrophile by converting them into tosyl leaving groups. The two segments are joined in dimethylformamide and unless the reactants are very diluted, ordinary polymerization will take place to long linear chains and not cyclization. In the final step the tosyl groups are removed with sulfuric acid.
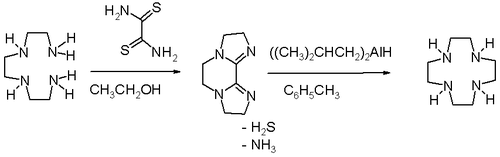
High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a bisamidine – also a bis(imidazoline) – followed by reduction and ring expansion with DIBAL.[3]
In one study [4] cyclen is covalently bonded through a propylene molecular spacer to adenine and chelated with zinc diperchlorate. This complex is able to selectively bind uracil and uridine in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evidenced by mass spectrometry.
See also
References
- ↑ Schrodt, Antje; Neubrand, Anton; Van Eldik, Rudi (1997). "Fixation of CO2 by Zinc(II) Chelates in Alcoholic Medium. X-ray Structures of {[Zn(cyclen)]3(μ3-CO3)}(ClO4)4 and [Zn(cyclen)EtOH](ClO4)2". Inorg. Chem. 36: 4579–4584. doi:10.1021/ic961368t.
- 1 2 Atkins, T. J.; Richman, J. E.; Oettle, W. F. (1988). "1,4,7,10,13,16-Hexaazacyclooctadecane". Org. Synth.; Coll. Vol., 6, p. 652
- ↑ Reed, David P.; Weisman, Gary R. (2004). "1,4,7,10-Tetraazacyclododecane". Org. Synth.; Coll. Vol., 10, p. 667
- ↑ Xia, Chuan-Qin; Tan, Xin-Yu; Chen, Shan-Yong; Yue, Yang; Yu, Xiao-Qi (2006). "The conjugate of adenine–cyclen Zn(II) complex: its synthesis and selective recognition abilities for uracil and uridine" (PDF). Arkivoc. 2: 68–76.
Further reading
- Suchý, M.; Hudson, R. H. E. (2008). "Synthetic Strategies Toward N-Functionalized Cyclens". Eur. J. Org. Chem. 2008 (29): 4847–4865. doi:10.1002/ejoc.200800636.