Bechamp reaction
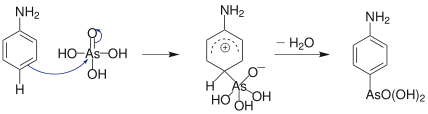
In organic synthesis the Bechamp reaction, first reported in 1863 by Antoine Béchamp,[1] is used for producing arsonic acids from activated aromatic rings; for example the synthesis of arsanilic acid from aniline.[2][3][4]
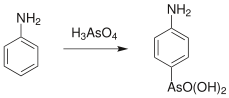
The reaction is an electrophilic aromatic substitution, using arsenic acid as the electrophile. Important products of this reaction include roxarsone; which exhibits an anticoccidial action and promotes growth in animals.[5]
References
- ↑ M. A. Bechamp (1863). "de l'action de la chaleur sur l'arseniate d'analine et de la formation d'un anilide de l'acide arsenique". Compt. Rend. 56: 1172–1175.
- ↑ P. Ehrlich & A. Bertheim, (1907). "Überp-Aminophenylarsinsäure". Chemische Berichte. 40 (3): 3292. doi:10.1002/cber.19070400397.
- ↑ H. P. Brown & C. S. Hamilton, (1934). "Naphthalenearsonic Acids. The Application of the Béchamp Reaction to α-Naphthylamine". J. Am. Chem. Soc. 56: 151. doi:10.1021/ja01316a047.
- ↑ C. S. Hamilton & J. F. Morgan (1944). "The Preparation of Aromatic Arsonic and Arsinic Acids by the Bart, Bechamp, and Rosenmund Reactions". Organic Reactions: 2. doi:10.1002/0471264180.or002.10. ISBN 0471264180.
- ↑ John F. Stolz; Eranda Perera; Brian Kilonzo; Brian Kail; Bryan Crable; Edward Fisher; Mrunalini Ranganathan; Lars Wormer & Partha Basu (2007). "Biotransformation of 3-Nitro-4-hydroxybenzene Arsonic Acid (Roxarsone) and Release of Inorganic Arsenic by Clostridium Species". Environ. Sci. Technol. 41 (3): 818–823. doi:10.1021/es061802i. PMID 17328188.
This article is issued from Wikipedia - version of the 6/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.