Damascone
Not to be confused with Damascenone.
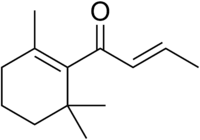 | |
| Names | |
|---|---|
| IUPAC name
(E)-1-(2,6,6-Trimethyl-1-cyclohexenyl)but-2-en-1-one | |
| Other names
Rose ketones | |
| Identifiers | |
| 23726-91-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4524302 |
| ECHA InfoCard | 100.041.660 |
| PubChem | 5374527 |
| |
| |
| Properties | |
| C13H20O | |
| Molar mass | 192.30 g/mol |
| Density | 0.934 g/mL |
| Hazards | |
| R-phrases | R43 |
| S-phrases | S36/37 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
| Names | |
|---|---|
| IUPAC name
(E)-1-(2,6,6-trimethyl-1-cyclohex-2-enyl)but-2-en-1-one | |
| Other names
Rose ketones | |
| Identifiers | |
| 24720-09-0 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.041.660 |
| |
| Properties | |
| C13H20O | |
| Molar mass | 192.30 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Damascones are a series of closely related chemical compounds that are components of a variety of essential oils. The damascones belong to a family of chemicals known as rose ketones, which also includes damascenones and ionones. beta-Damascone is a contributor to the aroma of roses, despite its relatively low concentration, and is an important fragrance chemical used in perfumery.[2]
The damascones are derived from the degradation of carotenoids.[3][4]
See also
References
- ↑ β-Damascone at Sigma-Aldrich
- ↑ Rose (Rosa damascena), John C. Leffingwell
- ↑ Biogeneration of C13-norisoprenoid compounds: experiments supportive for an apo-carotenoid pathway in grapevines. R. Baumes, J. Wirth, S. Bureau, Y. Gunata and A. Razungles, Analytica Chimica Acta, 29 April 2002, Volume 458, Issue 1, pages 3–14, doi:10.1016/S0003-2670(01)01589-6
- ↑ Volatile Compounds Released by Enzymatic Hydrolysis of Glycoconjugates of Leaves and Grape Berries from Vitis vinifera Muscat of Alexandria and Shiraz Cultivars. Jérémie Wirth, Wenfei Guo, Raymond Baumes and Ziya Günata, J. Agric. Food Chem., 2001, 49 (6), pages 2917–2923, doi:10.1021/jf001398l
Further reading
- Charles S. Sell. (2003). A fragrant introduction to terpenoid chemistry. Cambridge: RSC, Royal Society of Chemistry. pp. 256–257. ISBN 978-0-85404-681-2.
This article is issued from Wikipedia - version of the 9/23/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.