Binapacryl
 | |
| Names | |
|---|---|
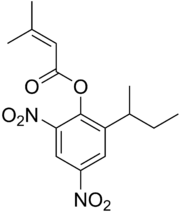
| IUPAC name
(RS)-(2-Butan-2-yl-4,6-dinitrophenyl) 3-methylbut-2-enoate | |
| Other names
Dapacryl; Morocide; Morrocid; Acricid; Endosan; Ambox; Dinoseb methacrylate | |
| Identifiers | |
| 485-31-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:82153 |
| ChemSpider | 9817 |
| ECHA InfoCard | 100.006.921 |
| KEGG | C19022 |
| PubChem | 10234 |
| UNII | 4X685BB13A |
| |
| |
| Properties | |
| C15H18N2O6 | |
| Molar mass | 322.32 g·mol−1 |
| Density | 1.2 g/cm3 |
| Melting point | 66 to 67 °C (151 to 153 °F; 339 to 340 K) |
| Insoluble | |
| Hazards | |
| R-phrases | R21/22 R50/53 R61 |
| S-phrases | S45 S53 S60 S61 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Binapacryl is an miticide and fungicide.[2] Chemically, it is an ester derivative of dinoseb. Although binapacryl has low toxicity itself, it is readily metabolized to form dinoseb, which is toxic.[1]
International trade in binapacryl is regulated by the Rotterdam Convention.
References
- 1 2 Datasheet from International Programme on Chemical Safety
- ↑ Binapacryl at alanwood.net
This article is issued from Wikipedia - version of the 11/1/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.