Blue bottle experiment
.webm.jpg)
The blue bottle experiment is a chemical reaction. An aqueous solution containing glucose, sodium hydroxide, methylene blue and some air is shaken in a closed bottle; it turns from colorless to blue and then decolorizes again after a while. With further shaking, the cycle can be repeated several times.[1] This experiment is a classic chemistry demonstration and can be used in laboratory courses as a general chemistry experiment. In terms of kinetics reaction based mechanism experiment.[2] The reaction will work with other reducing sugars besides glucose and also with other reducing dyes.
Classical version

The aqueous solution in the classical reaction contains glucose, sodium hydroxide and methylene blue. In the first step the enolate of glucose is formed. The next step is a redox reaction of the enolate with methylene blue. The glucose is oxidized to gluconic acid which, in alkaline solution is in the sodium gluconate form. Methylene blue is reduced to colorless leucomethylene blue. Pseudo first order reaction can be used to describe the result, as the aim is to understand the effects on the concentration over the course of the solution going back to becoming colorless from blue. [3]
If there is enough available oxygen, leucomethylene blue is then re-oxidized to methylene blue and the blue color of the solution is restored. The availability of oxygen is increased by shaking the solution. When the solution comes to rest, glucose reduction of the redox dye again takes the upper hand and the color of the solution disappears. The reaction is first order in glucose, methylene blue and hydroxide ion and zero-order in oxygen. Other glucose oxidation products besides sodium gluconate that are reported are D-arabino-hexos-2-ulose (glucosone), the anion of D-arabinonate after splitting of a formate anion and finally arabinonic acid.[4]
Green version
Wellman and Noble proposed a new formulation for the Blue Bottle experiment, they use ascorbic acid instead of glucose, while methylene blue and Oxygen are still used.[5] The chemical pattern formation of redox reaction in ascorbic acid follows as methylene blue is reduced by vitamin C under acidic conditions. Copper is used as a catalyst, for the reoxidation of leucomethylene blue to methylene blue. This is a great example for green chemistry as the chemical waste is reduced which in turn makes the condition less corrosive.[6]
Green versions of the chemical traffic light experiment and the vanishing valentine experiment is also present.
Air Oxidation of Ascorbic acid
Four (families) of dyes are used in the oxidation thiazines, oxazines, azines, and indigo carmine, reported to work with glucose and caustic soda. The pH is changed from ∼13 to ∼3 due to caustic soda to , which can be easily neutralized by half a teaspoon of baking soda before disposal. It is also noted that the observation of ascorbic acid is achieved in a wider range of pH.
Pattern formation
Pattern formation is when a solution containing NaOH, glucose, and dye is poured into an open petri dish which is open to the atmosphere. This will result in solution changing its structure over a period of time. Structures arise from molecular transport through diffusion and chemical kinetics. Patterns formed in the petri dish can be described as a mosaic pattern; web-like, dynamic spiral, branching, and lines connecting to each others.[7]
There are factors that can affect pattern formation. Changes in pattern formation are not homogeneous and can be caused by several factors. Different types of dye in solution will give the same pattern because of the bond's formation and the dynamics remain the same, this is because the solution has the same colour as the dye. Different amounts of dye can result in density change in the solution and this results in changing of convective motion. Different amounts of dye can bring in different amounts of convention cell which are also formed by different amounts of glucose and oxidized product. This can result in an interesting spatial phenomena. Time can also affect pattern formation. As the time passed, one pattern gradually faded away. Spirals and branches started to disappear and eventually disappeared fully.[8] These facts indicate that oxygen affects the chemical reaction and this plays a fundamental role in the pattern formation. Pattern formation may also form from a chemically driven convective instability. This means that matter is exchanged across the air-reaction mixture interface, due to the fluctuations in the molecular nature of chemical systems.[8]
A small group of researchers of the University of Glasgow named Pons, Batiste and Bees had came up with a small conclusion about pattern formation in the methylene blue-glucose system. They came up with a conclusive statement that a similar pattern can be formed in a container with accessible oxygen. This resulting surface tension effect isn't required to produce the instability. Small holes were also found in the lid of container that oxygen can't access resulting in a thin, blue, and lower amount of oxygen. Pattern length and time scale had been explored in one of their experiments due to the variation in viscosity and fluid depth. The experiment reveals that the wavelength is formed as a pattern starts to form quickly. Then wavelength or pattern can be maintained or oscillate for a while.[9]
-

web-liked started to form and solution is quite clear
-

spread of lines and dynamic spiral and solution is still clear
-
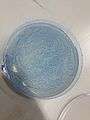
mosaic pattern and lines are all connected and solution is getting blue
-

dynamic pattern and branching are clearing out and solution is blue
-

Blue Bottle Experiment - color range
-

Equipments and Reagents used for the Blue Bottle Experiment
-

Blue Bottle Experiment - color range
See also
References
- ↑ Baker, Colin. "The 'blue bottle' reaction". Education in Chemistry. Royal Society of Chemistry. Retrieved 14 July 2016.
- ↑ C.Engerer and G. Cook (November 1, 1999). "The Blue Bottle Reaction as a General Chemistry Experiment on Reaction Mechanisms". Journal of Chemical Education. 11 (76): 1519. doi:10.1021/ed076p1519.
- ↑ Baker, Colin. "The 'blue bottle' reaction". Education in Chemistry. Royal Society of Chemistry. Retrieved 14 July 2016.
- ↑ What Is Happening When the Blue Bottle Bleaches: An Investigation of the Methylene Blue-Catalyzed Air Oxidation of Glucose, Laurens Anderson, Stacy M. Wittkopp, Christopher J. Painter, Jessica J. Liegel, Rodney Schreiner, Jerry A. Bell, and Bassam Z. Shakhashiri Journal of Chemical Education 2012 89 (11), 1425-1431 doi:10.1021/ed200511d
- ↑ Wellman and Noble, Whitney and Mark. "Greening the Blue Bottle". Journal of Chemical Education: p 537. doi:10.1021/ed080p537.
- ↑ Rajchakit and Limpanuparb, Urawadee and Taweetham (October 16, 2015). "Greening the Traffic Light: Air Oxidation of Vitamin C Catalyzed by Indicators". Journal of Chemical Education: 1486–1489. doi:10.1021/acs.jchemed.5b00630.
- ↑ Pattern Formation in the Methylene-Blue-Glucose System, Pons. A. J, Sague ́s. F, Bees. M. A, Graae Sørensen. P J. Phys. Chem. B 2000, 104 (10), 2251–2259 doi:10.1021/jp9935788
- 1 2 The Blue Bottle Experiment and Pattern Formation in this System, Adamčíková. L`, Ševčík. P Zeitschrift für Naturforschung A 52a, 650-654 (1997) doi:10.1515/zna-1997-8-918
- ↑ Nonlinear chemoconvection in the methylene-blue–glucose system: Two-dimensional shallow layers, Pons. A. J, Batiste. O, Bees. M. A Physical Review E 78, 016316 2008 doi:10.1103/PhysRevE.78.016316
External links
| Wikimedia Commons has media related to The blue bottle experiment. |