Carbon peapod
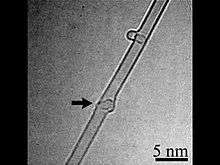


Carbon peapod is a hybrid nanomaterial consisting of spheroidal fullerenes encapsulated within a carbon nanotube. It is named due to their resemblance to the seedpod of the pea plant. Since the properties of carbon peapods differ from those of nanotubes and fullerenes, the carbon peapod can be recognized as a new type of a self-assembled graphitic structure.[4] Possible applications of nano-peapods include nanoscale lasers, single electron transistors, spin-qubit arrays for quantum computing, nanopipettes, and data storage devices thanks to the memory effects and superconductivity of nano-peapods.[5][6]
History
Single-walled nanotubes (SWNTs) were first seen in 1993 as cylinders rolled from a single graphene sheet. In 1998, the first peapod was observed by Brian Smith, Marc Monthioux and David Luzzi.[7] The idea of peapods came from the structure that was produced inside a transmission electron microscope in 2000.[4] They were first recognized in fragments obtained by a pulsed-laser vaporization synthesis followed by treatment with an acid and annealing.[8][9][10]
Production and structure
Carbon peapods can be naturally produced during carbon nanotube synthesis by pulsed laser vaporization. C60 fullerene impurities are formed during the annealing treatment and acid purification, and enter the nanotubes through defects or vapor-phase diffusion.[11] Fullerenes within a nanotube are only stabilized at a diameter difference of 0.34 nm or less, and when the diameters are nearly identical, the interacting energy heightens to such a degree (comparable to 0.1 GPa) that the fullerenes become unable to be extracted from the SWNT even under high vacuum.[4] The encapsulated fullerenes have diameters close to that of C60 and form a chain inside the tube. Controlled production of carbon peapods allow for greater variety in both the nanotube structure and the fullerene composition. Varying elements can be incorporated into a carbon peapod through doping and will dramatically affect the resulting thermal and electrical conductivity properties.
Chemical properties
The existence of carbon peapods demonstrates further properties of carbon nanotubes, such as potential to be a stringently controlled environment for reactions. C60 molecules normally form amorphous carbon when heated to 1000–1200 °C under ambient conditions; when heated to such a high temperature within a carbon nanotube, they instead merge in an ordered manner to form another SWNT, thus creating a double-wall carbon nanotube.[4] Owing to the ease with which fullerenes can encapsulate or be doped with other molecules and the transparency of nanotubes to electron beams, carbon peapods can also serve as nano-scale test tubes. After fullerenes containing reactants diffuse into an SWNT, a high-energy electron beam can be used to displace carbon atoms and induce high reactivity, thus triggering formation of C60 dimers and merging of their contents.[12] Additionally, due to the enclosed fullerenes being limited to only a one-dimensional degree of mobility, phenomena such as diffusion or phase transformations can easily be studied.[11]
Electronic properties
The diameter of carbon peapods range from ca. 1 to 50 nanometers. Various combinations of fullerene C60 sizes and nanotube structures can lead to various electric conductivity property of carbon peapods due to orientation of rotations. For example, the C60 @ (10,10) is a good superconductor and the C60 @ (17,0) peapod is a semiconductor. The calculated band gap of C60 @ (17,0) equals 0.1 eV.[13] Research into their potential as semiconductors is still ongoing. Although both the doped fullerides and ropes of SWNTs are superconductors, unfortunately, the critical temperatures for the superconducting phase transition in these materials are low. There are hopes that carbon nano-peapods could be superconducting at room temperature.[14]
With chemical doping, the electronic characteristics of peapods can be further adjusted. When carbon peapod is doped with alkali metal atoms like potassium, the dopants will react with the C60 molecules inside the SWNT. It forms a negatively charged C606− covalently bound, one-dimensional polymer chain with metallic conductivity. Overall, the doping of SWNTs and peapods by alkali metal atoms actively enhances the conductivity of the molecule since the charge is relocated from the metal ions to the nanotubes.[15] Doping carbon nanotubes with oxidized metal is another way to adjust conductivity. It creates a very interesting high temperature superconducting state as the Fermi level is significantly reduced. A good application would be the introduction of silicon dioxide to carbon nanotubes. It constructs memory effect as some research group has invented ways to create memory devices based on carbon peapods grown on Si/SiO2 surfaces.[16][17]
References
| Wikimedia Commons has media related to Carbon peapods. |
- ↑ Gorantla, Sandeep; Börrnert, Felix; Bachmatiuk, Alicja; Dimitrakopoulou, Maria; Schönfelder, Ronny; Schäffel, Franziska; Thomas, Jürgen; Gemming, Thomas; Borowiak-Palen, Ewa; Warner, Jamie H.; Yakobson, Boris I.; Eckert, Jürgen; Büchner, Bernd; Rümmeli, Mark H. (2010). "In situ observations of fullerene fusion and ejection in carbon nanotubes". Nanoscale. 2 (10): 2077–9. doi:10.1039/C0NR00426J. PMID 20714658.
- ↑ Gimenez-Lopez, Maria del Carmen; Chuvilin, Andrey; Kaiser, Ute; Khlobystov, Andrei N. (2011). "Functionalised endohedral fullerenes in single-walled carbon nanotubes". Chem. Commun. 47 (7): 2116–2118. doi:10.1039/C0CC02929G.
- ↑ Barzegar, Hamid Reza; Gracia-Espino, Eduardo; Yan, Aiming; Ojeda-Aristizabal, Claudia; Dunn, Gabriel; Wågberg, Thomas; Zettl, Alex (2015). "C60/Collapsed Carbon Nanotube Hybrids: A Variant of Peapods". Nano Letters. 15 (2): 829–34. doi:10.1021/nl503388f. PMID 25557832.
- 1 2 3 4 Iijima, Sumio (2002). "Carbon nanotubes: Past, present, and future". Physica B: Condensed Matter. 323: 1–5. Bibcode:2002PhyB..323....1I. doi:10.1016/S0921-4526(02)00869-4.
- ↑ Kwon, Young-Kyun; Tománek, David; Iijima, Sumio (1999). ""Bucky Shuttle" Memory Device: Synthetic Approach and Molecular Dynamics Simulations". Physical Review Letters. 82 (7): 1470–1473. Bibcode:1999PhRvL..82.1470K. doi:10.1103/PhysRevLett.82.1470.
- ↑ Utko, Pawel; Nygård, Jesper; Monthioux, Marc; Noé, Laure (2006). "Sub-Kelvin transport spectroscopy of fullerene peapod quantum dots". Applied Physics Letters. 89 (23): 233118. doi:10.1063/1.2403909.
- ↑ Pichler, T.; Kuzmany, H.; Kataura, H.; Achiba, Y. (2001). "Metallic Polymers of C60 Inside Single-Walled Carbon Nanotubes". Physical Review Letters. 87 (26). Bibcode:2001PhRvL..87z7401P. doi:10.1103/PhysRevLett.87.267401.
- ↑ Burteaux, Beatrice; Claye, Agnès; Smith, Brian W.; Monthioux, Marc; Luzzi, David E.; Fischer, John E. (1999). "Abundance of encapsulated C60 in single-wall carbon nanotubes". Chemical Physics Letters. 310: 21–24. Bibcode:1999CPL...310...21B. doi:10.1016/S0009-2614(99)00720-4.
- ↑ Smith, Brian W.; Monthioux, Marc; Luzzi, David E. (1998). "Encapsulated C60 in carbon nanotubes". Nature. 396 (6709): 323–324. doi:10.1038/24521.
- ↑ Smith, Brian W.; Monthioux, Marc; Luzzi, David E. (1999). "Carbon nanotube encapsulated fullerenes: A unique class of hybrid materials". Chemical Physics Letters. 315: 31–36. Bibcode:1999CPL...315...31S. doi:10.1016/S0009-2614(99)00896-9.
- 1 2 Smith, Brian W.; Luzzi, David E. (2000). "Formation mechanism of fullerene peapods and coaxial tubes: A path to large scale synthesis". Chemical Physics Letters. 321: 169–174. Bibcode:2000CPL...321..169S. doi:10.1016/S0009-2614(00)00307-9.
- ↑ Terrones, M (2010). "Transmission electron microscopy: Visualizing fullerene chemistry". Nature Chemistry. 2 (2): 82–3. doi:10.1038/nchem.526. PMID 21124394.
- ↑ Chen, Jiangwei; Dong, Jinming (2004). "Electronic properties of peapods: Effects of fullerene rotation and different types of tube". Journal of Physics: Condensed Matter. 16 (8): 1401–1408. doi:10.1088/0953-8984/16/8/021.
- ↑ Service, R. F. (2001). "SOLID-STATE PHYSICS: Nanotube 'Peapods' Show Electrifying Promise". Science. 292 (5514): 45. doi:10.1126/science.292.5514.45. PMID 11294210.
- ↑ Yoon, Young-Gui; Mazzoni, Mario S. C.; Louie, Steven G. (2003). "Quantum conductance of carbon nanotube peapods". Applied Physics Letters. 83 (25): 5217. doi:10.1063/1.1633680.
- ↑ Lee, C. H.; Kang, K. T.; Park, K. S.; Kim, M. S.; Kim, H. S.; Kim, H. G.; Fischer, J. E.; Johnson, A. T. (2003). "The Nano-Memory Devices of a Single Wall and Peapod Structural Carbon Nanotube Field Effect Transistor". Japanese Journal of Applied Physics. 42: 5392–5394. doi:10.1143/JJAP.42.5392.
- ↑ Krive, I. V.; Shekhter, R. I.; Jonson, M. (2006). "Carbon "peapods"—a new tunable nanoscale graphitic structure (Review)". Low Temperature Physics. 32 (10): 887. doi:10.1063/1.2364474.