Castanospermine
 | |
| Names | |
|---|---|
| IUPAC name
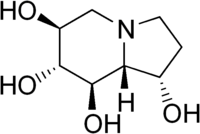
(1S,6S,7R,8R,8aR)-1,2,3,5,6,7,8,8a-octahydroindolizine-1,6,7,8-tetrol | |
| Identifiers | |
| 79831-76-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27860 |
| ChEMBL | ChEMBL311226 |
| ChemSpider | 49177 |
| ECHA InfoCard | 100.127.469 |
| PubChem | 54445 |
| |
| |
| Properties | |
| C8H15NO4 | |
| Molar mass | 189.209 g/mol |
| Appearance | White to off-white solid |
| Melting point | 212 to 215 °C (414 to 419 °F; 485 to 488 K) |
| Soluble | |
| Hazards | |
| R-phrases | R20/21/22 |
| S-phrases | S26 S36 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Castanospermine is an indolizidine alkaloid first isolated from the seeds of Castanospermum australe.[3] It is a potent inhibitor of some glucosidase enzymes[4] and has antiviral activity in vitro and in mouse models.[5]
Castanospermine was a lead to celgosivir.
See also
References
- ↑ Merck Index, 11th Edition, 1902.
- ↑ Castanospermine at Fermentek
- ↑ Hohenschutz, Liza D.; Bell, E. Arthur; Jewess, Phillip J.; Leworthy, David P.; Pryce, Robert J.; Arnold, Edward; Clardy, Jon (1981). "Castanospermine, a 1,6,7,8-tetrahydroxyoctahydroindolizine alkaloid, from seeds of Castanospermum australe". Phytochemistry. 20 (4): 811–14. doi:10.1016/0031-9422(81)85181-3.
- ↑ R Saul; J J Ghidoni; R J Molyneux & A D Elbein (1985). "Castanospermine inhibits alpha-glucosidase activities and alters glycogen distribution in animals". PNAS. 82 (1): 93–97. doi:10.1073/pnas.82.1.93. PMC 396977
 . PMID 3881759.
. PMID 3881759. - ↑ Whitby K, Pierson TC, Geiss B, Lane K, Engle M, Zhou Y, Doms RW, Diamond MS (2005). "Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo". J Virol. 79 (14): 8698–706. doi:10.1128/JVI.79.14.8698-8706.2005. PMC 1168722
 . PMID 15994763.
. PMID 15994763.
This article is issued from Wikipedia - version of the 9/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.