Chan-Lam coupling
| Chan-Lam coupling | |
|---|---|
| Named after | Dominic Chan Patrick Lam |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | chan-lam-coupling |
| RSC ontology ID | RXNO:0000374 |
The Chan–Lam coupling reaction – also known as the Chan–Evans–Lam coupling, as Chan, Evans, and Lam published their work back-to-back in Tetrahedron Letters – is a cross-coupling reaction between an aryl boronic acid and an alcohol or an amine to form the corresponding secondary aryl amines or aryl ethers, respectively. The Chan–Lam coupling is catalyzed in the presence of copper(II) species and may be conducted in air at room temperature. This is in contrast to the Buchwald–Hartwig coupling, which relies on the use of expensive noble metal catalysts (palladium), non-generalizable ligands, and a basic species (for deprotonation of the N-H and O-H bonds). In this regard, the Chan–Lam coupling has several benefits over other C-N and C-O coupling reactions. Because motifs containing aryl C-N and C-O bonds are frequently found in pharmaceuticals, the Chan–Lam coupling has seen a great deal of application.
Often stoichiometric copper is used, as only a small amount of the copper proceeds to a catalytically active species. The mechanism, which is still rather controversial, is shown in the below figure:
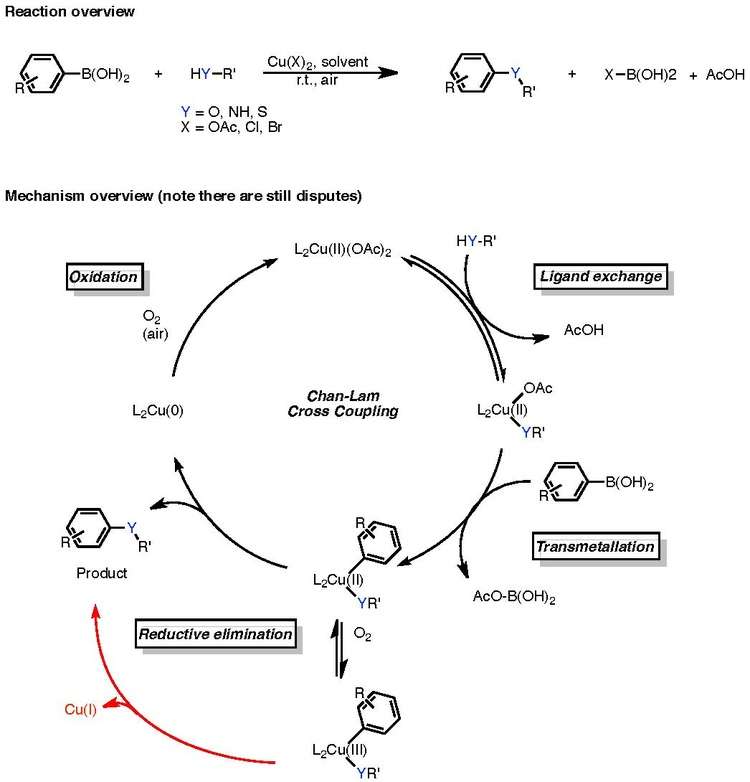
It is presently unknown whether the Chan-Lam coupling proceeds through a copper(II) to copper(0) catalytic cycle, or if it proceeds through a copper(II), copper(III), copper(I) process and then, in the presence of ambient air oxygen if this copper(I) species is then recycled to copper(0), restarting the cycle. Nevertheless, the catalytic cycle is characterized by de facto (I) ligand exchange, (II) transmetallation, (III) reductive elimination, and (IV) oxidation.
A recent example of the Chan–Lam coupling to synthesize biologically active compounds is shown below:

Compound 1, a pyrrolic amine, is coupled with aryl boronic ester, 2, to afford product 3, which is then carried forward to the biologically active target 4. The nitrile group of 2 does not poison the Cu(OAc)2 used, and pyridine is the ligand used for the reaction. Though the reaction is characterized by a lengthy duration (three days), it was carried out at room temperature in ambient air and resulted in a net 93% yield.
References
- https://www.princeton.edu/chemistry/macmillan/group-meetings/JLA_CopperMechanisticStudies.pdf
- New N- and O-Arylations with Phenylboronic acids and Cupric Acetate. Chan, Dominic; Monaco, Kevin; Wang, R.; Winter, Michael. Tetrahedron Lett. VOL 39 (1998) pp. 2933–2936
- Synthesis of Diaryl Ethers through the Copper-Promoted Arylation of Phenols with Arylboronic Acids. An Expedient Synthesis of Thyroxine. Evans, David; Katz, J.; West, T. Tetrahedron Lett. VOL 39 (1998) pp. 2937–2942
- New Aryl/Heteroaryl C-N Bond Cross-coupling Reactions via Arylboronic Acid/Cupric Acetate Arylation. Lam, Patrick; Clark, Charles; Saubern, Simon; Adams, Jessica; Winters, Michael; Chan, Dominic; Combs, Andrew Tetrahedron Lett. VOL. 39 (1998) PP. 2941-2944
- Copper-promoted/catalyzed C-N and C-O Bond Cross-coupling with Vinylboronic Acid and Its Utilities. Lam, Patrick; Bonne, Damien; Vincent, Guillaume; Clark, Charles. Tetrahedron Lett., VOL. 44 (2003) pp. 4927–4931.
- Copper Promoted C-N and C-O Bond Cross-coupling with Phenyl and Pyridylboronates. Chan, Dominic; Monaco, Kevin; Li, Renhua; Bonne, Damien; Clark, Charles; Lam, Patrick. Tetrahedron Lett., VOL.> 44 (2003) pp. 3863–3865.