chemicalize.org
|
| |
 The startpage of chemicalize.org | |
| Initial release | January 11, 2009 |
|---|---|
| Stable release |
0.11.0 beta
/ 22 March 2011 |
| Written in | Java, JavaScript, PHP |
| Platform | independent |
| Available in | English |
| Type | Science software, Search engine software |
| Website |
www |
chemicalize.org is a free chemical structure miner and web search engine developed and owned by ChemAxon. The main purpose of chemicalize.org is to identify chemical names (SMILES, traditional and IUPAC names) on websites and convert them to chemical structures. chemicalize.org provides other services such as structure based predictions, chemical search, and a “chemicalized” web search.
Short history of the tool's development
2009 Chemicalize a webpage
- creates a modified version of the current webpage, where chemical names in the page are extended with a structure image (denoted with dotted underline).
May 2010 Calculate properties
- provides a web interface for structure based predictions of several physical and chemical properties calculated by Marvin.[1] (See the list of predicted structure based properties below.)
November 2010 Chemical search
- in this service, the structure is a subject of substructure and/or similarity search, and each result shows all the websites where that structure was found.
March 2011 Web search
- Google Search query is generated with the chemical names on the input to find all relevant pages on the internet. The familiar Google Search result page is extended with the 2D image of all structures found on the resulting pages. Queries may contain multiple chemical names as well as non-chemical text.
- a chemical Table of Contents of structures found in the document is displayed at the top of the chemicalized Web pages.
List of the predicted structure based properties
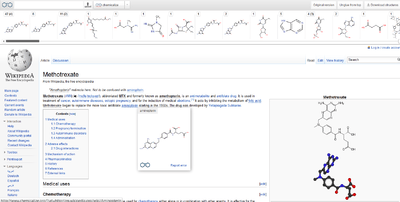
A "chemicalized" Wikipedia page with the table of content on the top, and with the tooltip above an underdotted chemical name.
- IUPAC name
- pKa,[2][3] logP and logD[4]
- Isoelectric point
- Charge
- Polarizability
- Orbital Electronegativity[5]
- Tautomerization
- Stereoisomer generation
- Topology Analysis
- Geometry data
- Polar Surface Area
- Molecular Surface Area in 3D
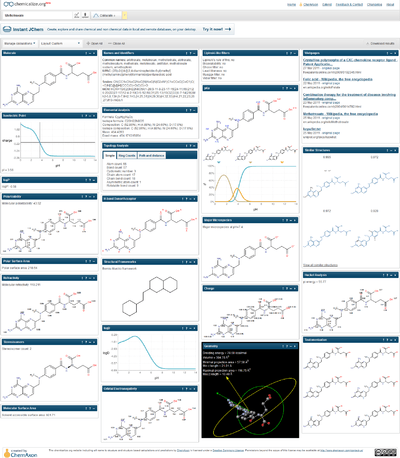
The chemical properties and calculations shown through the example of the methotrexate.
- Hydrogen bond Donor-Acceptor
- Huckel Analysis[6]
- Refractivity
- Structural Framework
- Resonance[7]
- Lipinski's Rule of Five
See also
References
- ↑ Bunin, Barry A. (2007). Chemoinformatics: theory, practice, & products. Springer. pp. 87–88.
- ↑ Dewick, Paul M. (2006). Essentials of organic chemistry:for students of pharmacy, medicinal chemistry and biological chemistry. John Wiley & Sons Ltd. p. 696.
- ↑ William O. Foye; Thomas L. Lemke; David A. Williams (2008). Foye's principles of medicinal chemistry. Walters Kluwer: Lippincott Williams & Wilkins. pp. 78–81.
- ↑ Mannhold, Raimond (2008). Molecular drug properties: measurement and prediction. Weinheim: Wiley-VHC Verlag GmbH & Co.KGaA. p. 426.
- ↑ Leach, Andrew L. (2001). Molecular modelling: principles and applications second edition. Pearson Education Limited. p. 193.
- ↑ Boeyens, Jan C. A. (2003). The theories of chemistry. Elsevier. p. 393.
- ↑ Wheland, George Willard (1944). Resonance in organic chemistry. J. Wiley and Sons, inc.
External links
- Changelog of chemicalize.org
- ChemSpider integrated chemicalize.org technology
- CCblog about the chemicalize.org's beta release
- The bbgm blog's article about the chemicalize.org by Deepak Singh
- ChemAxon extends chemicalize.org, article in Drug Discovery World
- Article on SYS-CON media
- chemicalize.org among Safety References of The good scents company
- Article on Fierce BioTech
- Drug Discovery Opinion article
This article is issued from Wikipedia - version of the 11/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.