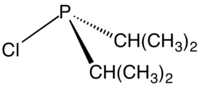
Chlorodiisopropylphosphine
 | |
| Identifiers | |
|---|---|
| 40244-90-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 469344 |
| ECHA InfoCard | 100.157.609 |
| PubChem | 538967 |
| |
| |
| Properties | |
| C6H14ClP | |
| Molar mass | 152.60 |
| Appearance | colorless liquid |
| Boiling point | 46-47° (10 mm of Hg) |
| Hazards | |
| Main hazards | toxic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chlorodiisopropylphosphine is an organophosphorus compound with the formula [(CH3)2CH]2PCl. It is a colorless liquid that is used to prepared phosphine ligands.
Synthesis and reactions
The compound is prepared by treating phosphorus trichloride with the Grignard reagent derived from isopropyl chloride:[1]
- PCl3 + 2 (CH3)2CHMgCl → [(CH3)2CH]2PCl + 2 MgCl2
Relative to the reaction of less hindered Grignard reagents with PCl3, this reaction affords a superior yield of the monochloro derivative.
Chlorodiisopropylphosphine reacts with alcohols and phenols to give phosphonites, this reaction typically is conducted in the presence of a base:
- [(CH3)2CH]2PCl + ROH → [(CH3)2CH]2POR + HCl
Phosphinites are versatile ligands.[2]
References
- ↑ W. Voskuil and J. F. Arens "Chlorodiisopropylphosphine" Org. Synth. 1968, volume 48, 47. doi:10.15227/orgsyn.048.0047
- ↑ for example: Pandarus, V., Zargarian, D., "New Pincer-Type Diphosphinito (POCOP) Complexes of Nickel", Organometallics 2007, volume 26, 4321. doi:10.1021/om700400x
This article is issued from Wikipedia - version of the 2/16/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.