Chlorophyll a
 | |
| Names | |
|---|---|
| IUPAC name
Chlorophyll a | |
| Systematic IUPAC name
Magnesium [methyl (3S,4S,21R)-14-ethyl-4,8,13,18-tetramethyl-20-oxo-3-(3-oxo-3-{[(2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-yl]oxy}propyl)-9-vinyl-21-phorbinecarboxylatato(2-)-κ2N,N'] | |
| Other names
α-Chlorophyll | |
| Identifiers | |
| 479-61-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 16736115 |
| ECHA InfoCard | 100.006.852 |
| EC Number | 207-536-6 |
| E number | E140 (colours) |
| PubChem | 6433192 |
| RTECS number | FW6420000 |
| UNII | YF5Q9EJC8Y |
| |
| |
| Properties | |
| C55H72MgN4O5 | |
| Molar mass | 893.51 g·mol−1 |
| Appearance | Green |
| Odor | Odorless |
| Density | 1.079 g/cm3[1] |
| Melting point | ~ 152.3 °C (306.1 °F; 425.4 K)[2] decomposes[1] |
| Insoluble | |
| Solubility | Very soluble in EtOH, ether Soluble in ligroin,[2] acetone, C6H6, CHCl3[1] |
| Absorbance | See text |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
Chlorophyll a is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light.[3] It also reflects green/yellow light, and as such contributes to the observed green color of most plants. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain.[4] Chlorophyll a also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located.[5]
 |  |
Distribution of chlorophyll a
Chlorophyll a is essential for most photosynthetic organisms to release chemical energy but is not the only pigment that can be used for photosynthesis. All oxygenic photosynthetic organisms use chlorophyll a, but differ in accessory pigments like chlorophylls b.[4] Chlorophyll a can also be found in very small quantities in the green sulfur bacteria, an anaerobic photoautotroph.[6] These organisms use bacteriochlorophyll and some chlorophyll a but do not produce oxygen.[6] Anoxygenic photosynthesis is the term applied to this process, unlike oxygenic photosynthesis where oxygen is produced during the light reactions of photosynthesis.
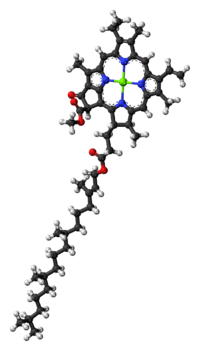
Molecular structure
The molecular structure of chlorophyll a consists of a chlorin ring, whose four nitrogen atoms surround a central magnesium atom, and has several other attached side chains and a hydrocarbon tail.
Chlorin ring

Chlorophyll a contains a magnesium ion encased in a large ring structure known as a chlorin. The chlorin ring is a heterocyclic compound derived from pyrrole. Four nitrogen atoms from the chlorin surround and bind the magnesium atom. The magnesium center uniquely defines the structure as a chlorophyll molecule.[7] The porphyrin ring of bacteriochlorophyll is saturated, and lacking alternation of double and single bonds causing variation in absorption of light.[8]
Side chains

Side chains are attached to the chlorin ring of the various chlorophyll molecules. Different side chains characterize each type of chlorophyll molecule, and alters the absorption spectrum of light.[9] [10] For instance, the only difference between Chlorophyll a and Chlorophyll b is that Chlorophyll b has an aldehyde instead of a methyl group at the C-7 position.[10]
Hydrocarbon tail
Chlorophyll a has a long hydrophobic tail, which anchors the molecule to other hydrophobic proteins in the thylakoid membrane of the chloroplast.[4] Once detached from the porphyrin ring, this long hydrocarbon tail becomes the precursor of two biomarkers, pristane and phytane. Both of which are important in the study of geochemistry and the determination of petroleum sources.
Biosynthesis
Chlorophyll a biosynthetic pathway utilizes a variety of enzymes.[11] Genes code for the enzymes on the Mg-tetrapyrroles of both bacteriochlorophyll a and chlorophyll a.[11] It begins with glutamic acid, which is transformed into a 5-aminolevulinic acid (ALA). Two molecules of ALA are then reduced to porphobilinogen (PBG), and four molecules of PBG are then coupled, forming protoporphyrin IX.[7] When forming protoporphyrin, Mg-chelatase acts as a catalyst for the insertion of Mg into the chlorophyll a structure.[11] The pathway then uses either a light-dependent process, driven by the enzyme protochlorophyllide oxidoreductase. Protochlorophyllide is a precursor to the production of a chlorophyll a molecule, or a light-independent process driven by other enzymes, to form a cyclic ring, and the reduction of another ring in the structure.[7] Attachment of the phytol tail completes the process of chlorophyll biosynthesis.[12]
Reactions of photosynthesis
Absorbance of light
Light spectrum
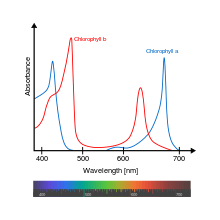
Chlorophyll a absorbs light within the violet, blue and red wavelengths while mainly reflecting green. This reflectance gives chlorophyll its green appearance. Accessory photosynthetic pigments broaden the spectrum of light absorbed, increasing the range of wavelengths that can be used in photosynthesis.[4] The addition of chlorophyll b next to chlorophyll a extends the absorption spectrum. In low light conditions, plants produce a greater ratio of chlorophyll b to chlorophyll a molecules, increasing photosynthetic yield.[9]
Light gathering
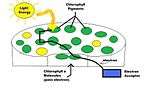
Absorption of light by photosynthetic pigments converts photons into chemical energy. Light energy radiating onto the chloroplast strikes the pigments in the thylakoid membrane and excites their electrons. Since the chlorophyll a molecules only capture certain wavelengths, organisms may use accessory pigments to capture a wider range of light energy shown as the yellow circles.[5] It then transfers captured light from one pigment to the next as resonance energy, passing energy one pigment to the other until reaching the special chlorophyll a molecules in the reaction center.[9] These special chlorophyll a molecules are located in both photosystem II and photosystem I. They are known as P680 for Photosystem II and P700 for Photosystem I.[13] P680 and P700 are the primary electron donors to the electron transport chain.
Primary electron donation
Chlorophyll a is very important in the energy phase of photosynthesis. Two electrons need to be passed to an electron acceptor for the process of photosynthesis to proceed.[4] Within the reaction centers of both photosystems there are a pair of chlorophyll a molecules that pass electrons on to the transport chain through redox reactions.[13]
See also
- Photosystem II light harvesting protein
- Chlorophyll c, an accessory pigment of chlorophyll
- Chlorophyll b, another related chemical
References
- 1 2 3 Anatolievich, Kiper Ruslan. "chlorophyll a". http://chemister.ru. Retrieved 2014-08-23. External link in
|website=(help) - 1 2 Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ PHOTOSYNTHESIS
- 1 2 3 4 5 Raven, Peter H.; Evert, Ray F.; Eichhorn, Susan E. (2005). "Photosynthesis, Light, and Life". Biology of Plants (7th ed.). W.H. Freeman. pp. 119–127. ISBN 0-7167-9811-5.
- 1 2 Papageorgiou,G, and Govindjee (2004). "Chlorophyll a Fluorescence, A Signature of Photosynthesis". 19. Springer: 14,48,86
- 1 2 Eisen JA, Nelson KE, Paulsen IT, et al. (July 2002). "The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium". Proc. Natl. Acad. Sci. U.S.A. 99 (14): 9509–14. doi:10.1073/pnas.132181499. PMC 123171
 . PMID 12093901. See pages 9514,48,86.
. PMID 12093901. See pages 9514,48,86.
- 1 2 3 Zeiger, Eduardo; Taiz, Lincoln (2006). "Ch. 7: Topic 7.11: Chlorophyll Biosynthesis". Plant physiology (4th ed.). Sunderland, Mass: Sinauer Associates. ISBN 0-87893-856-7.
- ↑ Campbell, Mary K.; Farrell, Shawn O. (20 November 2007). Biochemistry (6th ed.). Cengage Learning. p. 647. ISBN 978-0-495-39041-1.
- 1 2 3 Lange, L.; Nobel, P.; Osmond, C.; Ziegler, H. (1981). Physiological Plant Ecology I – Responses to the Physical Environment. 12A. Springer-Verlag. pp. 67, 259.
- 1 2 Niedzwiedzki, Dariusz M.; Blankenship, Robert E. (December 2010). "Singlet and triplet excited state properties of natural chlorophylls and bacteriochlorophylls". Photosynthesis Research. 106 (3): 227–238. doi:10.1007/s11120-010-9598-9. PMID 21086044.
- 1 2 3 Suzuki JY, Bollivar DW, Bauer CE (1997). "Genetic Analysis of Chlorophyll biosynthesis." (PDF). Annu. Rev. Genet. 31 (1): 61–89. doi:10.1146/annurev.genet.31.1.61.
- ↑ Zeiger & Taiz 2006, Figure 7.11.A: The biosynthetic pathway of chlorophyll
- 1 2 Ishikita H, Saenger W, Biesiadka J, Loll B, Knapp EW (June 2006). "How photosynthetic reaction centers control oxidation power in chlorophyll pairs P680, P700, and P870". Proc. Natl. Acad. Sci. U.S.A. 103 (26): 9855–60. doi:10.1073/pnas.0601446103. PMC 1502543
 . PMID 16788069.
. PMID 16788069.
External links
- Zeiger & Taiz 2006, Topic 7.11: Chlorophyll Biosynthesis