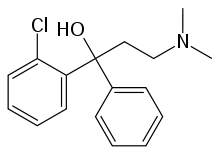
Clofedanol
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Oral |
| ATC code | R05DB10 (WHO) |
| Identifiers | |
| |
| CAS Number |
791-35-5 |
| PubChem (CID) | 2795 |
| IUPHAR/BPS | 7324 |
| DrugBank |
DB04837 |
| ChemSpider |
2693 |
| UNII |
42C50P12AP |
| KEGG |
D07721 |
| ChEMBL |
CHEMBL1201313 |
| ECHA InfoCard | 100.011.219 |
| Chemical and physical data | |
| Formula | C17H20ClNO |
| Molar mass | 289.8 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Clofedanol (INN) or chlophedianol (BAN) is a centrally acting cough suppressant used in the treatment of dry cough. Clofedanol has local anesthetic and antihistamine properties, and may have anticholinergic effects at high doses.[1] It is marketed in Canada under the trade name Ulone, but is not available currently in the United States. In August 2009, Centrix Pharmaceutical announced the launch of a cough syrup containing a mixture of clophedianol and pseudoephedrine, marketed under the brand name Clofera. It has a release to market date in the beginning of the fourth quarter 2009.[2] Chlophedianol was approved for OTC status in 1987 by the FDA OTC monograph process[3] and its safety and efficacy data are limited.
Synthesis

Clofedanol synthesis: R. Lorenz and H. Henecka, U.S. Patent 3,031,377 (1962).
See also
References
- ↑ "Clofedanol" (in French). BIAM. 1998-07-24. Retrieved 2007-04-15.
- ↑ "Centrix Pharmaceutical Announces Clofera(TM), a Unique Antitussive and Nasal Decongestant for the Temporary Relief of Cough and Nasal Congestion". Cenrx.com. Retrieved 2009-09-27.
- ↑ "Department of Health and Human Services. Food and Drug Administration. 21 CFR Parts 310, 341, and 369. Docket No. 76N-052T. Cold, cough, allergy, bronchodilator, and antiasthmatic drug products for over-the0counter human use; final monograph for OTC antitussive drug products. Federal Register 1987;52(155):30042-57" (PDF). FDA.gov. 1987-08-12.
This article is issued from Wikipedia - version of the 4/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.