Congo red
 | |
 | |
| Names | |
|---|---|
| IUPAC name
disodium 4-amino-3-[4-[4-(1-amino-4-sulfonato-naphthalen-2-yl)diazenylphenyl]phenyl]diazenyl-naphthalene-1-sulfonate | |
| Identifiers | |
| 573-58-0 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL429694 |
| ChemSpider | 10838 |
| ECHA InfoCard | 100.008.509 |
| MeSH | Congo+red |
| PubChem | 11313 |
| UNII | 3U05FHG59S |
| |
| |
| Properties | |
| C32H22N6Na2O6S2 | |
| Molar mass | 696.665 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Congo red is the sodium salt of 3,3′-([1,1′-biphenyl]-4,4′-diyl)bis(4-aminonaphthalene-1-sulfonic acid) (formula: C32H22N6Na2O6S2; molecular weight: 696.66 g/mol). It is a secondary diazo dye. Congo red is water-soluble, yielding a red colloidal solution; its solubility is better in organic solvents such as ethanol.
It has a strong, though apparently noncovalent, affinity to cellulose fibers. However, the use of Congo red in the cellulose industries (cotton textile, wood pulp, and paper) has long been abandoned, primarily because of its toxicity and tendency to run and change color when touched by sweaty fingers.
History
Congo red was first synthesized in 1883 by Paul Böttiger, who was working then for the Friedrich Bayer Company in Elberfeld, Germany. He was looking for textile dyes that did not require a mordant step. The company was not interested in this bright red color, so he filed the patent under his name and sold it to the AGFA company of Berlin. AGFA marketed the dye under the name "Congo red", a catchy name in Germany at the time of the 1884 Berlin West Africa Conference, an important event in the Colonisation of Africa. The dye was a major commercial success for AGFA. In the following years, for the same reasons, other dyes were marketed using the "Congo" name: Congo rubine, Congo corinth, brilliant Congo, Congo orange, Congo brown, and Congo blue.[1]
Behavior in solution
| Congo red (pH indicator) | ||
| below pH 3.0 | above pH 5.2 | |
| 3.0 | ⇌ | 5.2 |
Due to a color change from blue to red at pH 3.0–5.2, Congo red can be used as a pH indicator. Since this color change is an approximate inverse of that of litmus, it can be used with litmus paper in a simple parlor trick: add a drop or two of Congo red to both an acid solution and a base solution. Dipping red litmus paper in the red solution will turn it blue, while dipping blue litmus paper in the blue solution will turn it red. This property gives Congo red a metachromatic property as a dye, both in strongly acidic solutions and with strongly acidophilic tissue.
Congo red has a propensity to aggregate in aqueous and organic solutions. The proposed mechanisms suggest hydrophobic interactions between the aromatic rings of the dye molecules, leading to a pi–pi stacking phenomenon. Although these aggregates are present under various sizes and shapes, the "ribbon-like micelles" of a few molecules seem to be the predominant form (even if the "micelle" term is not totally appropriate here). This aggregation phenomenon is more prevalent in high Congo red concentrations, at high salinity and/or low pH.
Diagnostic use
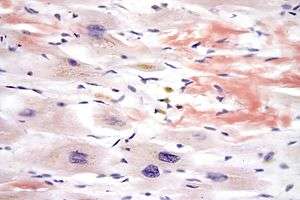
In biochemistry and histology, Congo red is used to stain microscopic preparates, especially as a cytoplasm and erythrocyte stain. Apple-green birefringence of Congo red stained preparates under polarized light is indicative for the presence of amyloid fibrils. Additionally, Congo red is used in microbiological epidemiology to rapidly identify the presence of virulent serotype 2a Shigella flexneri, where the dye binds the bacterium's unique lipopolysaccharide structure. Furthermore, Congo red may also be used as an artificial inducer of Shigella flexneri type III secretion needles, bringing about the secretion of various virulence proteins such as IpaB and IpaC, which form the translocation pore within the host cell membrane, allowing effector proteins to pass through and alter the biochemistry of the cell.
References
- ↑ Steensma, D. P. (2001). "Congo Red: Out of Africa?" (pdf). Archives of Pathology and Laboratory Medicine. 125 (2): 250–252. doi:10.1043/0003-9985(2001)125<0250:CR>2.0.CO;2. PMID 11175644.