Corrin
 | |
| Identifiers | |
|---|---|
| 262-76-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:33221 |
| ChemSpider | 16736705 |
| PubChem | 6438343 |
| |
| |
| Properties | |
| C19H22N4 | |
| Molar mass | 306.40478 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
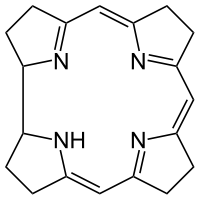
Corrin is an heterocyclic compound. It is the parent macrocycle related to substituted derivative that is found in vitamin B12. Its name reflects that it is the "core" of vitamin B12 (cobalamins).[1]
Coordination chemistry
Upon deprotonation, the corrinoid ring is capable of binding cobalt. In vitamin B12, the resulting complex also features a benzimidazole-derived ligand, and the sixth site on the octahedron serves as the catalytic center.
The corrin ring resembles the porphyrin ring, which occurs in hemoglobin. Both feature four pyrrole-like subunits organized into a ring. In contrast to porphyrins, corrins lack one of the carbon groups that link the pyrrole-like units. Thus the large ring has 19 carbons, whereas porphyrins have 20. Also, the pyrrole-like rings in corrin are fully saturated "edge-carbon" centers. Because of the high number of sp3 carbon centers, corrins are more flexible than porphyrins and are not as flat. They do not have a full conjugated character around the entire ring. Instead, the ring has a kind of "3/4" conjugation.
Corroles (octadehydrocorrins) are fully aromatic derivatives of corrins.
References
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.