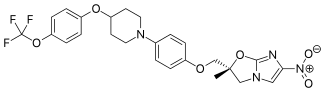
Delamanid
 | |
| Clinical data | |
|---|---|
| Trade names | Deltyba |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (film-coated tablets) |
| ATC code | J04AK06 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ≥99.5% |
| Metabolism |
in plasma by albumin, in liver by CYP3A4 (to a lesser extent) |
| Biological half-life | 30–38 hours |
| Excretion | not excreted in urine[1] |
| Identifiers | |
| |
| Synonyms | OPC-67683 |
| CAS Number | 681492-22-8 |
| PubChem (CID) | 6480466 |
| ChemSpider | 4981055 |
| ChEMBL | CHEMBL218650 |
| Chemical and physical data | |
| Formula | C25H25F3N4O6 |
| Molar mass | 534.48 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Delamanid (USAN, INN) is a drug for the treatment of multi-drug-resistant tuberculosis. It works by blocking the synthesis of mycolic acids in Mycobacterium tuberculosis, the organism which causes tuberculosis, thus destabilising its cell wall.[2][3][4] The drug is approved in the EU under the trade name Deltyba (made by Otsuka Pharmaceutical).
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[5]
Adverse effects
Delamanid prolongs QT interval.[6]
Interactions
Delamanid is metabolised by the liver enzyme CYP3A4, wherefore strong inducers of this enzyme can reduce its effectiveness.[6]
History
In phase II clinical trials, the drug was used in combination with standard treatments, such as four or five of the drugs ethambutol, isoniazid, pyrazinamide, rifampicin, aminoglycoside antibiotics, and quinolones. Healing rates (measured as sputum culture conversion) were significantly better in patients who additionally took delamanid.[4][7]
The European Medicines Agency (EMA) recommended conditional marketing authorization for delamanid in adults with multidrug-resistant pulmonary tuberculosis without other treatment options because of resistance or tolerability. The EMA considered the data show that the benefits of delamanid outweigh the risks, but that additional studies were needed on the long-term effectiveness.[8]
See also
- Pretomanid, a related drug
References
- ↑ "Deltyba (delamanid): Summary of Product Characteristics. 5.2. Pharmacokinetic Properties" (PDF). Otsuka Novel Products GmbH. p. 10. Retrieved 9 July 2016.
- ↑ Matsumoto, M.; Hashizume, H.; Tomishige, T.; Kawasaki, M.; Tsubouchi, H.; Sasaki, H.; Shimokawa, Y.; Komatsu, M. (2006). "OPC-67683, a Nitro-Dihydro-Imidazooxazole Derivative with Promising Action against Tuberculosis in Vitro and in Mice". PLoS Medicine. 3 (11): e466. doi:10.1371/journal.pmed.0030466. PMC 1664607
 . PMID 17132069.
. PMID 17132069. - ↑ Skripconoka, V.; Danilovits, M.; Pehme, L.; Tomson, T.; Skenders, G.; Kummik, T.; Cirule, A.; Leimane, V.; Kurve, A.; Levina, K.; Geiter, L. J.; Manissero, D.; Wells, C. D. (2012). "Delamanid Improves Outcomes and Reduces Mortality for Multidrug-Resistant Tuberculosis". European Respiratory Journal. 41 (6): 1393–1400. doi:10.1183/09031936.00125812. PMC 3669462
 . PMID 23018916.
. PMID 23018916. - 1 2 H. Spreitzer (18 February 2013). "Neue Wirkstoffe – Bedaquilin und Delamanid". Österreichische Apothekerzeitung (in German) (4/2013): 22.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- 1 2 Pharmazeutische Zeitung: Delamanid: Neuer Wirkstoff gegen multiresistente TB, 9 May 2014. (German)
- ↑ Gler, M. T.; Skripconoka, V.; Sanchez-Garavito, E.; Xiao, H.; Cabrera-Rivero, J. L.; Vargas-Vasquez, D. E.; Gao, M.; Awad, M.; Park, S. K.; Shim, T. S.; Suh, G. Y.; Danilovits, M.; Ogata, H.; Kurve, A.; Chang, J.; Suzuki, K.; Tupasi, T.; Koh, W. J.; Seaworth, B.; Geiter, L. J.; Wells, C. D. (2012). "Delamanid for Multidrug-Resistant Pulmonary Tuberculosis". New England Journal of Medicine. 366 (23): 2151–2160. doi:10.1056/NEJMoa1112433. PMID 22670901.
- ↑ Drug Discovery & Development. EMA Recommends Two New Tuberculosis Treatments. November 22, 2013.