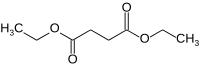
Diethyl succinate
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Diethyl butanedioate | |
| Other names
Diethyl succinate Butanedioic acid diethyl ester Clorius | |
| Identifiers | |
| 123-25-1 | |
| 3D model (Jmol) | Interactive image |
| 907645 | |
| ChemSpider | 13865630 |
| ECHA InfoCard | 100.004.194 |
| PubChem | 31249 |
| RTECS number | WM7400000 |
| |
| |
| Properties | |
| C8H14O4 | |
| Molar mass | 174.194 g/mol |
| Appearance | Colorless liquid |
| Density | 1.047 g/mL |
| Melting point | −20 °C (−4 °F; 253 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| Slightly soluble | |
| Vapor pressure | 0.13 mmHg |
| Thermochemistry | |
| Std enthalpy of combustion (ΔcH |
24.22 kJ/g |
| Hazards | |
| Main hazards | Primary irritant |
| NFPA 704 | |
| Flash point | 90.56 °C (195.01 °F; 363.71 K) |
| Explosive limits | 1.1-6.5% |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diethyl succinate is the diethyl ester of succinate.
Properties
Diethyl succinate is a colorless liquid with the formula C8H14O4. The organic molecule contains two ester groups and occurs naturally in both plant and animal tissues. Diethyl succinate plays a key role in the Krebs cycle which is a component of metabolism. Since it is low in molecular weight, this ester is commonly used in fragrances.[1]
Occurrences
Diethyl succinate is a byproduct of the fermentation of sugar. It is often present in fermented wines and beers that have aged a long time. Diethyl succinate is also present in the citric acid cycle.[2] The ester bond acts a good electron donor due to resonance, and therefore diethyl succinate acts in the electron transport chain. Diethyl succinate is also present as an additive in foods and synthetic flavorings and aromas.
Production
Diethyl succinate is formed during an esterification reaction between a carboxylic acid and an alcohol. [3]
- RCOOH + R'OH ↔ RCOOR' + water
Fisher esterification is a common method of synthesis in which the carboxylic acid reacts with an alcohol in the presence of a dehydrating reagent. The dehyrating reagent removes water from the reaction thus pushing the equilibrium toward producing more ester.[4] This takes advantage of le Chatelier's principles.
For diethyl succinate, the carboxylic acid involved is succinic acid, or butanedioic acid, which is a dicarboxyllic acid. The alcohol involved is ethanol.
Reactions
Diethyl succinate is produced through the reduction of a conjugated alkene with chromium(II) sulfate.[5]
Safety
Diethyl succinate can act as an irritant. If exposed to eyes, eyes should be flushed with water. Medical attention should be sought if symptoms occur. Upon skin contant, skin should be washed with soap and water.[6]
References
- ↑ Loudon, Marc (2009). Organic Chemistry. Colorado: Roberts. pp. A–4. ISBN 978-0-9815194-3-2.
- ↑ Lehman, John (2009). Operational Organic Chemistry. New Jersey: Pearson Education. p. 856. ISBN 0-13-600092-4.
- ↑ Cox, Douna, O'Donnell, Michael, Jennifer, Michael (2012). Molecular Biology. New York: W.H. Freeman. p. 633. ISBN 978-0-7167-7998-8.
- ↑ "Chemical Book". Chemical Book. Retrieved 2012-10-19.
- ↑ Orjuela, Kolah, Hong, Lira, Miller, Alvaro, Aspi, Xi, Carl, Dennis (2012). "Diethyl succinate synthesis by reactive distillation". Separation and Purification Technology. 88: 218. doi:10.1016/j.seppur.2011.11.033. Retrieved 2012-10-19.
- ↑ "Diethyl succinate". Sigma Aldrch. Retrieved 2012-10-19.
