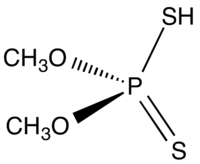
Dimethyl dithiophosphoric acid
 | |
| Names | |
|---|---|
| IUPAC name
Dimethoxy-sulfanyl-sulfanylidene-λ5-phosphane | |
| Other names
O,O-Dimethyl dithiophosphoric acid; Dimethyl dithiophosphate; Dimethyl phosphorodithioate; Dimethyl ester of phosphorodithioic acid | |
| Identifiers | |
| 756-80-9 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 12419 |
| EC Number | 212-053-9 |
| PubChem | 12959 |
| |
| |
| Properties | |
| C2H7O2PS2 | |
| Molar mass | 158.17 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 62–64 °C (144–147 °F; 335–337 K) 0.5 mm Hg |
| Hazards | |
| EU classification (DSD) |
Flammable (F) Toxic (T) Corrosive (C) |
| R-phrases | R23/24/25 R34 R41 |
| S-phrases | S23 S26 S36/37/39 S45 |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethyl dithiophosphoric acid is the organophosphorus compound with the formula (CH3O)2PS2H. It is the processor for production of the organothiophosphate insecticide Malathion. Although samples can appear dark, the compound is a colorless, distillable liquid.[1]
It is prepared by treating phosphorus pentasulfide with methanol:[2]
- P2S5 + 4 CH3OH → 2 (CH3O)2PS2H + H2S
References
- ↑ J. Svara, N. Weferling, T. Hofmann "Phosphorus Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006. doi:10.1002/14356007.a19_545.pub2
- ↑ Lefferts, J. L.; Molloy, K. C.; Zuckerman, J. J.; Haiduc, I.; Guta, C.; Ruse, D., "Oxy and thio phosphorus acid derivatives of tin. 1. Triorganotin(IV) dithiophosphate esters", Inorganic Chemistry 1980, volume 19, 1662-1670. doi:10.1021/ic50208a046
This article is issued from Wikipedia - version of the 5/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
