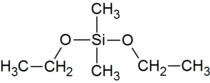
Dimethyldiethoxysilane
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Dimethyldiethoxysilane | |
| Systematic IUPAC name
Diethoxydimethylsilane | |
| Identifiers | |
| 78-62-6 | |
| 3D model (Jmol) | Interactive image Interactive image |
| Abbreviations | DMDEOS |
| 1736110 | |
| ChemSpider | 56117 |
| ECHA InfoCard | 100.001.025 |
| EC Number | 201-127-6 |
| PubChem | 62322 |
| RTECS number | VV3590000 |
| UN number | 2380 |
| |
| |
| Properties | |
| C6H16O2Si | |
| Molar mass | 148.28 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 0.865 g cm−3 |
| Melting point | −87 °C (−125 °F; 186 K) |
| Boiling point | 114 °C (237 °F; 387 K) |
| Solubility | soluble in carbon tetrachloride[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dimethyldiethoxysilane, sometimes abbreviated DMDEOS or DMDES, is an organosilicon compound. DMDEOS is a precursor in the production of the silicone polymer polydimethylsiloxane (PDMS).
DMDEOS is an intermediate silane useful for blocking hydroxyl and amino groups in organic synthesis reactions. This silylating step allows subsequent reactions to be carried out which would be adversely affected by the presence of active hydrogen in the hydroxyl or amine groups. Following the reaction step, hydroxyl or amine groups blocked with DMDEOS may be recovered by a hydrolysis procedure. DMDEOS is also used for preparing hydrophobic and release materials as well as enhancing flow of powders.[2] [3]
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–180, ISBN 0-8493-0594-2
- ↑ Merhari, Lhadi (2009), Hybrid Nanocomposites for Nanotechnology, Springer, pp. 184–185, ISBN 978-0-387-72398-3, retrieved 2009-07-19
- ↑ http://www.gpcsilicones.com/silanes/exp49.html
This article is issued from Wikipedia - version of the 9/23/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.