Diphenylzinc
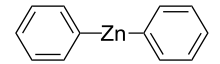 | |
 | |
| Identifiers | |
|---|---|
| 1078-58-6 | |
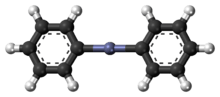
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.012.803 |
| |
| Properties | |
| C12H10Zn | |
| Molar mass | 219.59 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diphenylzinc is an organozinc compound. It is commonly used as the synthetic equivalent of a Ph− synthon. Solvent-free diphenylzinc exists as dimeric PhZn(μ-Ph)2ZnPh molecules in solid state.[1]
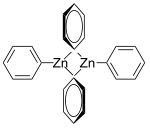
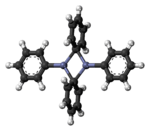
The dimeric solid state form of diphenylzinc.
Diphenylzinc is commercially available. It may be prepared by reaction of phenyllithium with zinc bromide:[2]
- 2 PhLi + ZnBr2 → Ph2Zn + 2 LiBr
It may also be prepared by the reaction of diphenylmercury with zinc metal.[3]
References
- ↑ Markies, Peter R.; Schat, Gerrit; Akkerman, Otto S.; Bickelhaupt, Friedrich; Smeets, Wilberth J. J.; Spek, Anthony L. (1990). "Coordinational behavior of solvent-free diorganylzinc compounds: the remarkable x-ray structure of dimeric diphenylzinc". Organometallics. 9 (8): 2243. doi:10.1021/om00158a022.
- ↑ Curtin, David Y.; Tveten, John L. (1961). "Reaction of Diarylzinc Reagents with Aryldiazonium Salts. Direct Formation of cis-Azo Compounds". J. Org. Chem. 26 (6): 1764. doi:10.1021/jo01065a017.
- ↑ Markies, P; Schat, Gerrit; Akkerman, Otto S.; Bickelhaupt, F.; Spek, Anthony L. (1992). "Complexation of diphenylzinc with simple ethers. Crystal structures of the complexes Ph2Zn·glyme and Ph2Zn·diglyme". J. Organomet. Chem. 430: 1. doi:10.1016/0022-328X(92)80090-K.
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.