Disulfur decafluoride
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Disulfur decafluoride | |||
| Systematic IUPAC name
Decafluoro-1λ6,2λ6-disulfane | |||
| Other names
sulfur pentafluoride | |||
| Identifiers | |||
| 5714-22-7 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 56348 | ||
| ECHA InfoCard | 100.024.732 | ||
| EC Number | 227-204-4 | ||
| MeSH | Disulfur+decafluoride | ||
| PubChem | 62586 | ||
| |||
| |||
| Properties | |||
| S2F10 | |||
| Molar mass | 254.1 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | like sulfur dioxide[1] | ||
| Density | 2.08 g/cm3 | ||
| Melting point | −53 °C (−63 °F; 220 K) | ||
| Boiling point | 30.1 °C (86.2 °F; 303.2 K) | ||
| insoluble[2] | |||
| Vapor pressure | 561 mmHg (20 °C)[1] | ||
| Hazards | |||
| Main hazards | Poisonous | ||
| NFPA 704 | |||
| Lethal dose or concentration (LD, LC): | |||
| LC50 (median concentration) |
2000 mg/m3 (rat, 10 min) 1000 mg/m3 (mouse, 10 min) 4000 mg/m3 (rabbit, 10 min) 4000 mg/m3 (guinea pig, 10 min) 4000 mg/m3 (dog, 10 min)[3] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 0.025 ppm (0.25 mg/m3)[1] | ||
| REL (Recommended) |
C 0.01 ppm (0.1 mg/m3)[1] | ||
| IDLH (Immediate danger) |
1 ppm[1] | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
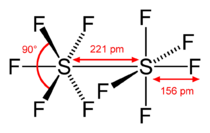
Disulfur decafluoride (S2F10) is a gas discovered in 1934 by Denbigh and Whytlaw-Gray.[4] Each S of the S2F10 molecule is octahedral, and surrounded by 5 fluorines.[5] S2F10 is highly toxic, with toxicity 4 times that of phosgene. It was considered a potential chemical warfare pulmonary agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. It is produced by the electrical decomposition of sulfur hexafluoride (SF6)—an essentially inert insulator used in high voltage systems such as transmission lines, substations and switchgear. S2F10 is also made during the production of SF6, but is distilled out.
Production
Disulfur decafluoride is produced primarily by the decomposition of sulfur hexafluoride:
2 SF6 → S2F10 + F2
Properties
This compound contains sulfur in the sp3d2 hybridization where 2 sulfur atoms have zero polarization between them and 5 polarized connections with fluorine atoms.
At temperatures above 150 °C, S
2F
10 decomposes slowly to SF
6 and SF
4:
S
2F
10 reacts with N
2F
4 to give SF
5NF
2. It reacts with SO
2 to form SF
5OSO
2F in the presence of ultraviolet radiation.
In the presence of excess chlorine gas, S
2F
10 reacts to form sulfur chloride pentafluoride (SF
5Cl):
- S
2F
10 + Cl
2 → 2 SF
5Cl
The analogous reaction with bromine is reversible and yields SF
5Br.[6] The reversibility of this reaction can be used to synthesize S
2F
10 from SF
5Br.[7]
Ammonia is oxidised by S
2F
10 into NSF
3.[8]
Toxicity
Disulfur decafluoride is a colorless gas or liquid with a sulfur-dioxide-like odor.[9] It is about 4 times as poisonous as phosgene. Its toxicity is thought to be caused by its disproportionation in the lungs into SF
6, which is inert, and SF
4, which reacts with moisture to form sulfurous acid and hydrofluoric acid.[10] Disulfur decafluoride itself is not toxic due to hydrolysis products, since it is hardly hydrolysed by water and most aqueous solutions.
External links
- "Sulfur Pentaflu". 1988 OSHA PEL Project. CDC NIOSH.
References
- 1 2 3 4 5 "NIOSH Pocket Guide to Chemical Hazards #0579". National Institute for Occupational Safety and Health (NIOSH).
- ↑ http://www.chemicalbook.com/ChemicalProductProperty_EN_CB0751782.htm
- ↑ "Sulfur pentafluoride". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Denbigh, K. G.; Whytlaw-Gray, R. (1934). "The Preparation and Properties of Disulphur Decafluoride". Journal of the Chemical Society. 1934: 1346–1352. doi:10.1039/JR9340001346.
- ↑ Harvey, R. B.; Bauer, S. H. (June 1953). "An Electron Diffraction Study of Disulfur Decafluoride". Journal of the American Chemical Society. 75 (12): 2840–2846. doi:10.1021/ja01108a015.
- ↑ Cohen, B.; MacDiarmid, A. G. (December 1965). "Chemical Properties of Disulfur Decafluoride". Inorganic Chemistry. 4 (12): 1782–1785. doi:10.1021/ic50034a025.
- ↑ Winter, R.; Nixon, P.; Gard, G. (January 1998). "A new preparation of disulfur decafluoride". Journal of Fluorine Chemistry. 87 (1): 85–86. doi:10.1016/S0022-1139(97)00096-1.
- ↑ Mitchell, S. (1996). Biological Interactions of Sulfur Compounds. CRC Press. p. 14. ISBN 0-7484-0245-4.
- ↑ "Sulfur Pentaflu". 1988 OSHA PEL Project. CDC NIOSH.
- ↑ Johnston, H. (2003). A Bridge not Attacked: Chemical Warfare Civilian Research During World War II. World Scientific. pp. 33–36. ISBN 981-238-153-8.
- Christophorou, L. G.; Sauers, I. (1991). Gaseous Dielectrics VI. Plenum Press. ISBN 0-306-43894-1.


