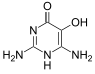
Divicine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,6-Diamino-4,5-dihydroxypyrimidine | |||
| Other names
2,6-Diamino-4,5-pyrimidinediol; 2,6-diamine-5-hydroxy-4(3H)-pyrimidinone; 2,4-diamino-5,6-dihydroxypyrimidine | |||
| Identifiers | |||
| 32267-39-3 | |||
| 3D model (Jmol) | (pyrimidine tautomer): Interactive image (pyrimidinone tautomer): Interactive image | ||
| ChemSpider | 2971290 | ||
| PubChem | 91606 | ||
| |||
| |||
| Properties | |||
| C4H6N4O2 | |||
| Molar mass | 142.12 g·mol−1 | ||
| Appearance | Brownish needles | ||
| Solubility in 10% KOH | Soluble | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Divicine (2,6-diamino-4,5-dihydroxypyrimidine) is an oxidant and a base with alkaloidal properties found in fava beans and Lathyrus sativus. It is an aglycone of vicine. A common derivative is the diacetate form (2,6-diamino-1,6-dihydro-4,5-pyrimidinedione).[1]
Occurrence
Divicine is found in fava beans and in the legume Lathyrus sativus, also known as khesari, which is a cheap and robust food source commonly grown in Asia and East Africa.
Synthesis
In plants, reduced divicine is formed from the hydrolysis of the inactive β–glucoside, vicine.[2]
A simplified three-step process for artificial divicine synthesis: (1) The benzyl group of 2-amino-5-benzyloxy-4-hydroxypyrimidine is removed by acid hydrolysis, yielding 2-amino-4,5-dihydroxypyrimidine. (2) This intermediate is then treated with nitrous acid to yield the slightly soluble orange product, 2-amino-6-nitrosopyrimidine-4,5-diol (3) which is then reduced with sodium dithionite to yield divicine.[3]
Toxicity
Divicine has been deemed a hemotoxic component of fava beans and plays a role in the development of favism, a disorder that involves a hemolytic response to the consumption of broad beans due to glucose-6-phosphate dehydrogenase (G6PD or G6PDH) deficiency. This deficiency, an X-linked recessive hereditary disease, is the most common enzyme deficiency worldwide. It is particularly common in those of African, Asian, Mediterranean, and Middle-Eastern descent. Symptoms of favism include hemolysis, prolonged jaundice, kernicterus, and even acute renal failure in extreme cases.[4]
The specific mechanism of divicine’s toxicity is still unknown. It had been established that the β–glucosides vicine and convicine were linked to the precipitation of hemolytic crises in G6PDH-deficient individuals,[5] but in a more recent study of rat erythrocyte toxicity, exposure to 1.5 mM of divicine dramatically reduced survival rates while exposure to 5 mM of vicine did not. These results suggest that divicine is a direct-acting hemolytic agent and likely the direct cause of favism.[6]
Divicine is also present in and at least partially responsible for the poisonous action of Lathyrus sativus - a legume commonly grown in drought- and famine-prone regions of Asia and East Africa as an ‘insurance crop’ for human consumption and livestock feed when other crops fail to grow, despite their known health hazards.[7]
References
- ↑ Bendich, C. (1953). Biochim. Biophys. Acta. 12: 462. Missing or empty
|title=(help) - ↑ Baker, M.; Bosia, A. (1984). "Mechanism of Action of Divicine in a Cell-free System and in Glucose-6-phosphate Dehydrogenase-deficient Red Cells". Toxicol. Pathol. 12: 331–336. doi:10.1177/019262338401200405.
- ↑ Chesterfield, J.; et al. (1964). "194. Pyrimidines. Part XIII. Electrophilic substitution at position 6 and a synthesis of divicine (2,4-diamino-5,6-dihydroxypyrimidine)". J. Chem. Soc.: 1001–1005. doi:10.1039/jr9640001001.
- ↑ Frank, J. (2005). "Diagnosis and management of G6PD deficiency". Am. Fam. Physician. 72: 1277–1282.
- ↑ Doyle, M. Ellin (1995). Food Safety 1995. Marcel Dekker, Inc. p. 357. ISBN 0-8247-9624-1.
- ↑ McMillan, D.; et al. (2001). "Favism: Effect of Divicine on Rat Erythrocyte Sulfhydryl Status, Hexose Monophosphate Shunt Activity, Morphology, and Membrane Skeletal Proteins". Toxicol. Sci. 62: 353–359. doi:10.1093/toxsci/62.2.353. PMID 11452148.
- ↑ http://www.biology-online.org/dictionary/Divicine