Elemene
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
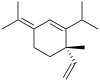
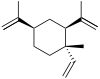
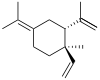
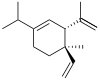
(+)-α: (S)-1-Isopropyl-6-methyl-3-(propan-2-ylidene)-6-vinylcyclohex-1-ene (−)-β: (1S,2S,4R)-1-Methyl-2,4-di(prop-1-en-2-yl)-1-vinylcyclohexane (−)-γ: (3R,4R)-1-Isopropyl-4-methyl-3-(prop-1-en-2-yl)-4-vinylcyclohex-1-ene (−)-δ: (3R,4R)-1-Isopropyl-4-methyl-3-(prop-1-en-2-yl)-4-vinylcyclohex-1-ene | |||
| Identifiers | |||
| 11029-06-4 (unspecified isomer) 5951-67-7 ((+)-α) 33880-83-0 ((±)-β) 515-13-9 ((−)-β) 29873-99-2 ((−)-γ) 20307-84-0 ((−)-δ) | |||
| 3D model (Jmol) | (+)-α: Interactive image (−)-β: Interactive image (−)-γ: Interactive image (−)-δ: Interactive image | ||
| PubChem | 80048 (α) 6918391 (β) | ||
| |||
| Properties | |||
| C15H24 | |||
| Molar mass | 204.36 g·mol−1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Elemenes are a group of closely related natural chemical compounds found in a variety of plants. The elemenes, which include α-, β-, γ-, and δ-elemene, are structural isomers of each other and are classified as sesquiterpenes. The elemenes contribute to the floral aromas of some plants,[1][2][3] and are used as pheromones by some insects.[4]
β-Elemene has attracted scientific interest because of its prevalence in a variety of medicinal plants. Experiments performed in vitro show that β-elemene has anti-proliferative effects toward some cancer cell types,[5][6][7][8] indicating the possibility of its use in chemotherapy. Small scale, low quality[9] clinical trials in China have been conducted in which benefits for cancer treatment have been reported. However, the Memorial Sloan–Kettering Cancer Center states that "human trials conducted so far are of poor quality".[10] A Cochrane Review of the available literature concluded that "there is no evidence from randomised controlled trials to confirm or refute the effectiveness of elemene as a treatment for lung cancer".[11]
References
- ↑ "Floral Compound: alpha-elemene". Pherobase.com.
- ↑ "Floral Compound: delta-elemene". Pherobase.com.
- ↑ "Floral Compound: gamma-elemene". Pherobase.com.
- ↑ "Semiochemical: beta-elemene". Pherobase.com.
- ↑ Zhu, T; Xu, Y; Dong, B; Zhang, J; Wei, Z; Xu, Y; Yao, Y (2011). "Β-elemene inhibits proliferation of human glioblastoma cells through the activation of glia maturation factor β and induces sensitization to cisplatin". Oncology Reports. 26 (2): 405–13. doi:10.3892/or.2011.1276. PMID 21519795.
- ↑ Yao, YQ; Ding, X; Jia, YC; Huang, CX; Wang, YZ; Xu, YH (2008). "Anti-tumor effect of beta-elemene in glioblastoma cells depends on p38 MAPK activation". Cancer Letters. 264 (1): 127–34. doi:10.1016/j.canlet.2008.01.049. PMID 18442668.
- ↑ Wang, G; Li, X; Huang, F; Zhao, J; Ding, H; Cunningham, C; Coad, JE; Flynn, DC; et al. (2005). "Antitumor effect of beta-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death". Cellular and Molecular Life Sciences. 62 (7–8): 881–93. doi:10.1007/s00018-005-5017-3. PMID 15868411.
- ↑ Li, X; Wang, G; Zhao, J; Ding, H; Cunningham, C; Chen, F; Flynn, DC; Reed, E; Li, QQ (2005). "Antiproliferative effect of beta-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase". Cellular and Molecular Life Sciences. 62 (7–8): 894–904. doi:10.1007/s00018-005-5027-1. PMID 15868412.
- ↑ Peng X, Zhao Y, Liang X, Wu L, Cui S, Guo A, Wang W (Feb 2006). "Assessing the quality of RCTs on the effect of beta-elemene, one ingredient of a Chinese herb, against malignant tumors". Contemp Clin Trials. 27 (1): 70–82. doi:10.1016/j.cct.2005.07.002. PMID 16243588.
- ↑ "About Herbs, Botanicals & Other Products: Beta-elemene". Memorial Sloan–Kettering Cancer Center.
- ↑ Peng, X; Zhao, Y; Liang, X; Wu, L; Cui, S; Guo, A; Wang, W (2006). "Assessing the quality of RCTs on the effect of beta-elemene, one ingredient of a Chinese herb, against malignant tumors". Contemporary Clinical Trials. 27 (1): 70–82. doi:10.1016/j.cct.2005.07.002. PMID 16243588.