Enediyne
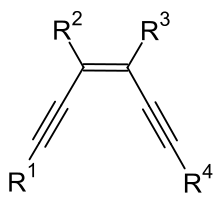

Enediynes are organic compounds containing two triple bonds and one double bond. The term is usually used to describe a class of bacterial natural products characterized by either nine- and ten-membered rings in which the double bond lies between the two triple bonds.[1] Many of these compounds are capable of undergoing Bergman cyclization. The resulting diradical, a 1,4-dehydrobenzene derivative, is capable of abstracting hydrogen atoms from the sugar backbone of DNA which results in strand scission.[2] This high reactivity with DNA makes the substances toxic. They are being researched as antitumor agents. Several of the naturally occurring enediynes have entered clinical trials against cancer[3] and in Japan neocarzinostatin is used clinically.
Three classes of enediynes exist:
The enediyne group in those compounds is often called a warhead.[4]
References
- ↑ K. C. Nicolaou; A. L. Smith; E. W. Yue (1993). "Chemistry and biology of natural and designed enediynes". PNAS. 90 (13): 5881–5888. Bibcode:1993PNAS...90.5881N. doi:10.1073/pnas.90.13.5881. PMC 46830
 . PMID 8327459.
. PMID 8327459. - ↑ S. Walker; R. Landovitz; W.D. Ding; G.A. Ellestad; D. Kahne (1992). "Cleavage behavior of calicheamicin gamma 1 and calicheamicin T". Proc Natl Acad Sci USA. 89 (10): 4608–12. Bibcode:1992PNAS...89.4608W. doi:10.1073/pnas.89.10.4608. PMC 49132
 . PMID 1584797.
. PMID 1584797. - ↑ Jones, G. B.; Fouad, F. S. (1 January 2004). "Designed Enediyne Antitumor Agents". Frontiers in Medicinal Chemistry - Online. 1 (1): 189–214. doi:10.2174/1567204043396640.
- ↑ Jones, G.; Fouad, F. (1 December 2002). "Designed Enediyne Antitumor Agents". Current Pharmaceutical Design. 8 (27): 2415–2440. doi:10.2174/1381612023392810.