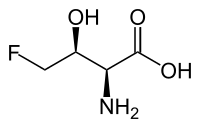
4-Fluoro-L-threonine
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3S)-2-Amino-4-fluoro-3-hydroxybutanoic acid | |
| Other names
4-Fluorothreonine | |
| Identifiers | |
| 102130-93-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 113553 |
| PubChem | 128057 |
| |
| |
| Properties | |
| C4H8FNO3 | |
| Molar mass | 137.11 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Fluoro-L-threonine is an antibacterial made by Streptomyces cattleya. It is formed by the fluorothreonine transaldolase catalysed transfer of fluoroacetaldehyde onto threonine.[1]
References
- ↑ Murphy CD, O'Hagan D, Schaffrath C (2001). "Identification of a PLP-Dependent Threonine Transaldolase: A Novel Enzyme Involved in 4-Fluorothreonine Biosynthesis in Streptomyces cattleya This work was supported by the Biotechnological and Biological Sciences Research Council and the University of St Andrews". Angew. Chem. Int. Ed. Engl. 40 (23): 4479–4481. doi:10.1002/1521-3773(20011203)40:23<4479::AID-ANIE4479>3.0.CO;2-1. PMID 12404452.
This article is issued from Wikipedia - version of the 5/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.