Fluxapyroxad
 | |
| Names | |
|---|---|
| IUPAC name
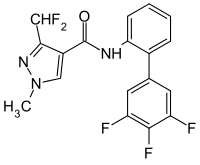
3-(difluoromethyl)-1-methyl-N-[2-(3',4',5'-trifluorophenyl)phenyl]pyrazole-4-carboxamide | |
| Other names | |
| Identifiers | |
| 907204-31-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:83113 |
| ChemSpider | 17253690 |
| PubChem | 16095400 |
| UNII | 7U8P4NAR2S |
| |
| |
| Properties | |
| C18H12F5N3O | |
| Molar mass | 381.31 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fluxapyroxad is a broad-spectrum pyrazole-carboxamide fungicide used on a large variety of commercial crops.[2][3] It stunts fungus growth by inhibiting the succinate dehydrogenase (SQR) enzyme.[3][4] Application of fluxapyroxad helps prevent many wilts and other fungal infections from taking hold. As with other systemic pesticides that have a long chemical half-life, there are concerns about keeping fluxapyroxad out of the groundwater, especially when combined with pyraclostrobin.[1] There is also concern that some fungi may develop resistance to fluxapyroxad.[5][6]
Biological action
Fluxapyroxad is a succinate dehydrogenase inhibitor (SDHI).[5] It interferes with a number of key fungal life functions, including spore germination, germ tube growth, appresoria formation and mycelium growth.[2] Specifically it interferes with the production of succinate dehydrogenase, the complex II in the mitochondrial respiration chain, which in turn interferes with the tricarboxylic cycle and mitochondrial electron transport.
Crops
Fluxapyroxad is commonly used as a fungicide for grains, row crops, vegetable crops, and fruit trees (pome and prunus), including:[7][8][9]
- Grains:
- Barley[10]
- Corn (all types)
- Oats
- Rice
- Rye
- Triticale
- Wheat
- Row and vegetable crops:
- Shelled peas and beans, both succulent and dried
- Edible-pod legume vegetables
- Fruiting vegetables (including tomatoes)
- Oilseed crops (flax seed, rapeseed, safflower and sunflower)
- Peanuts
- Soybeans
- Sugar beets
- Tuberous and corm vegetables (potato)
- Fruit trees:
- Apples
- Crabapples
- Oriental pears (Pyrus pyrifolia)
- Pears
- Apricots
- Cherries (sweet and tart)
- Nectarines
- Peaches
- Plums (all varieties)
- Nut trees
- Almond
- Pecan
Fungal diseases
Fluxapyroxad provides protection against many fungal diseases.[11] Studies have shown specific efficacy against diseases such as black point, Botrytis gray mold,[12] early blight,[13] and powdery mildew;[14] however, fluxapyroxad was found to have no efficacy against anthracnose on lentils.[15]
Toxicity
Fluxapyroxad has a low toxicity for humans, slightly toxic after a single ingestion, and relatively non-toxic after single inhalation or topical skin contact. However, fluxapyroxad is highly toxic to fish, fresh-water and salt-water invertebrates, and to aquatic plants, as well as being toxic to small mammals.[3][16][17] The primary target organ for fluxapyroxad exposure is the liver.[7] As the dose or duration of exposure to fluxapyroxad increased, clinical chemistry changes related to liver function also occurred, followed by hepatocellular necrosis, neoplastic changes in the liver, and tumors.[7] Fluxapyroxad was found "not likely" to be carcinogenic in humans and there was no evidence of neurotoxicity.[7]
The United States Environmental Protection Agency has established tolerance amounts that are allowed to be present on consumer food. These range from 0.05 ppm on almonds and pecans to 3.0 ppm on leafy brassica, and 15 ppm on other leafy vegetables.[7]
Registration and approval
Fluxapyroxad has been approved for use as a fungicide in the United States, Canada and the European Union.[18] In the spring of 2012, fluxapyroxad, trademarked under the names Sercadis®,[19] Imbrex®[20] and Xemium®[21] and manufactured by BASF Corporation, was registered for use as a fungicide in the United States. Fluxapyroxad is also one of the two active ingredients in Priaxor® fungicide and Merivon® fungicide, the other active ingredient being a strobilurin called pyraclostrobin.[22]
References
- 1 2 Office of Chemical Safety and Pollution Prevention, United States Environmental Protection Agency (2 May 2012). "Pesticide Fact Sheet: Fluxapyroxad" (PDF). Archived (PDF) from the original on 8 July 2013.
- 1 2 Strathmann, S.; Walker, S.; Barnes, J. (2011). "Fluxapyroxad: A new broad-spectrum fungicide". Phytopathology. 101 (6): 172.abstract
- 1 2 3 "Fluxapyroxad". New Active Ingredient Review. Minnesota Department of Agriculture. July 2012. Archived from the original on 25 June 2013.
- ↑ BASF, Crop Protection. "Xemium Fungicide Information". BASF Crop Protection. Retrieved March 2013. Check date values in:
|access-date=(help) - 1 2 "SDHI Fungicides". Fungicide Resistance Action Committee. Archived from the original on 22 June 2013.
- ↑ Veloukas, Thomas; Markoglou, Anastasios N.; Karaoglanidis, George S. (2013). "Differential Effect of SdhB Gene Mutations on the Sensitivity to SDHI Fungicides in Botrytis cinerea". Plant Disease. 97 (1): 118–122. doi:10.1094/pdis-03-12-0322-re. abstract
- 1 2 3 4 5 United States Environmental Protection Agency (26 February 2014). "Fluxapyroxad; Pesticide Tolerances". Federal Register.
- ↑ BASF, Crop Protection. "Priaxor Fungicide Product Label, Specimen, NVA 2013-04-372-0088" (PDF). BASF Crop Protection. Archived (PDF) from the original on 25 October 2014.
- ↑ BASF, Crop Protection. "Merivon Fungicide Product Sheet" (PDF). Archived (PDF) from the original on 25 October 2014.
- ↑ Coating barley seed with fluxapyroxad increased germination due to reduced fungal activity. Marquet, Nicolas (2012). "Nouvelles substances à la CIMA, la part belle aux SDHI". Phytoma-La Défense des végétaux. 659: 31–34. abstract
- ↑ "In-Field Research Shows Disease Control, Yield Advantages of Priaxor Fungicide and Merivon Fungicide From BASF". PMN Crop News. Plant Management Network. 27 March 2012. Archived from the original on 28 April 2012.
- ↑ Amiri, A.; Heath, S. M.; Peres, N. A. (2012). "Sensitivity of Botrytis cinerea field isolates to the novel succinate dehydrogenase inhibitors fluopyram, penthiopyrad, and fluxapyroxad". Phytopathology. 102 (7 (supplement)): S4.4. Abstract
- ↑ Gudmestad, Neil C.; et al. (2013). "Prevalence and Impact of SDHI Fungicide Resistance in Alternaria solani". Plant Disease. 97 (7): 952–960. doi:10.1094/PDIS-12-12-1176-RE. Abstract
- ↑ "Powdery Mildew on Peaches". Tree Fruit IPM Advisory. Utah State University. 6 May 2011. Archived from the original on 15 May 2011.
- ↑ Wunsch, Michael. "Recommendations for optimizing the control of anthracnose on lentils with fungicides" (PDF). NDSU Carrington Research Extension Center, North Dakota State University. Archived (PDF) from the original on 25 October 2014.
- ↑ "Safety Data Sheet Priaxor (4.0)" (PDF). Crop Data Management Systems, Inc. 29 September 2014. Archived (PDF) from the original on 25 October 2014.
- ↑ "Plant Disease Control". Guide to Field Crop Protection (PDF). Carman, Manitoba: Manitoba Agriculture, Food and Rural Development (MAFRD), Government of Manitoba. 2014. pp. 307–452, page 352. Archived (PDF) from the original on 25 October 2014.
- ↑ "BASF's new fungicide fluxapyroxad got EU approval". Archived from the original on 24 July 2012.
- ↑ EPA Registration Number 7969-309
- ↑ EPA Registration Number 7969-306
- ↑ EPA Registration Number 7969-308
- ↑ BASF, Newsroom. "New Priaxor fungicide and Merivon fungicide now registered for use". BASF Crop Protection.