Gamendazole
 | |
 | |
| Names | |
|---|---|
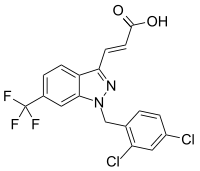
| IUPAC name
(E)-3-[1-[(2,4-Dichlorophenyl)methyl]-6-(trifluoromethyl)indazol-3-yl]prop-2-enoic acid[1] | |
| Other names
trans-3-(1-Benzyl-6-(trifluoromethyl)-1H-indazol-3-yl)acrylic acid) | |
| Identifiers | |
| 877773-32-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:90703 |
| ChemSpider | 9387234 |
| PubChem | 11212172 |
| |
| |
| Properties | |
| C18H11Cl2F3N2O2 | |
| Molar mass | 415.19 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Gamendazole is a novel drug candidate for male contraception. It is an indazole carboxylic acid derived from lonidamine (LND).
It has been shown to reduce fertility in male rats without affecting testosterone levels.
Rat studies
Gamendazole produced 100% antispermatogenic effects at 25 mg/kg i.p. in rats, whereas 200 mg/kg was fatal for 60% of rats tested. Since gamendazole produced 100% efficacy, it was tested orally. At a dose of 6 mg/kg, 100% of rats were infertile 4 weeks after a single administration. Complete infertility was maintained for 2 weeks, followed by complete recovery in 4 of 7 rats. The other 3 never recovered fertility. Upon dosing 6 mg/kg orally for 7 days, it produced similar infertility results, but only 2 of 7 rats recovered fertility. There were no abnormalities in rates of conception or abnormal conception in rats who recovered fertility.[2]
Pathology reports were conducted on gamendazole treated rats. At 25 mg/kg i.p., 6 mg/kg oral, and in animals that survived 200 mg/kg i.p., there were no remarkable findings, with no evidence of inflammation, necrosis, tumors, or hemorrhage. There was also a lack of observable behavioral effects at 25 mg/kg i.p., 6 mg/kg oral, and in animals that survived 200 mg/kg i.p. Gamendazole treatment had no effect on testosterone levels, and was reported to affect Sertoli cell function, leading to decreased levels of inhibin B. Low levels of inhibin B were correlated to the infertility of the rat.[2]
References
- ↑ "Gamendazole". NextBio. www.nextbio.com. Retrieved 31 July 2011.
- 1 2 Tash, Joseph (July 2008). "A Novel Potent Indazole Carboxylic Acid Derivative Blocks Spermatogenesis and Is Contraceptive in Rats after a Single Oral Dose". Biology of Reproduction. 78 (6): 1127–1138. doi:10.1095/biolreprod.106.057810. PMID 18218612.