Homocapsaicin
 | |
| Names | |
|---|---|
| IUPAC name
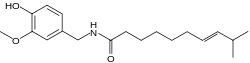
(6E)-N-(4-Hydroxy-3-methoxybenzyl)-8-methyldec-6-enamide | |
| Other names
Homocapsaicin II, N-Vanillyl-8-methyldec-6-(E)-enamide, trans-N-Vanillyl-8-methyldec-6-enamide, N-(4-Hydroxy-3-methoxybenzyl)-8-methyldec-trans-6-enamide, Vanillylamide of 8-methyldec-trans-6-enoic acid, HC | |
| Identifiers | |
| 71240-51-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 9848876 |
| PubChem | 11674147 |
| |
| |
| Properties | |
| C19H29NO3 | |
| Molar mass | 319.43 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
| Homocapsaicin | |
|---|---|
|
| |
| Heat | Above Peak (SR: 8,600,000) |
Homocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (Capsicum). Like capsaicin it is an irritant. Homocapsaicin accounts for about 1% of the total capsaicinoids mixture and has about half the pungency of capsaicin. Pure homocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. On the Scoville scale it has 8 600 000 SHU (Scoville heat units). Homocapsaicin isolated from chili pepper has been found in two isomeric forms, both with a carbon-carbon double bond at the 6 position (numbered from the amide carbon) on the 10-carbon acyl chain. One isomer has an additional carbon, a methyl group, at the 8 position and the other has a methyl group at the 9 position. Homocapsaicin (6-ene-8-methyl) is the more abundant isomer. Homocapsaicin with the double bond at the 7 position has never been found in nature, though its structure is widely reported on the Internet and, unfortunately, even in the scientific literature. Details of the misidentification have been published.[1] (Note: the structure to the right is incorrect and has not yet been edited.)
See also
- Capsaicin
- Dihydrocapsaicin
- Nordihydrocapsaicin
- Homodihydrocapsaicin
- Scoville scale
- Pepper spray
- Spice
References
- ↑ Thompson, R.Q. “Homocapsaicin: nomenclature, indexing, and identification” Flavour and Fragrance Journal 2007, 22, 243-248.