Iberulite
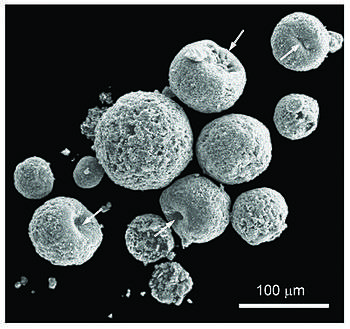
Iberulites are a particular type of microspherulites that develop in the atmosphere (troposphere), finally falling to the earth's surface. The name comes from the Iberian Peninsula where they were discovered.[1]
Definition
An iberulite is a co-association [note 1] with axial geometry, consisting of well-defined mineral grains, together with non-crystalline compounds, structured around a coarse-grained core with a smectite rind, only one vortex and pinkish color, formed in the troposphere by complex aerosol-water-gas interactions.
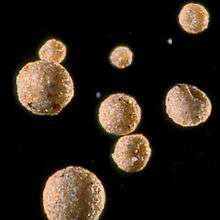
Formation
Shape
The aqueous interphase hypothesis has been suggested as the mechanism for tropospheric formation of iberulites:[1][3] interactions between water droplets and Saharan aerosols create complex hydrodynamic conditions [4] causing the possibility of collisions (wake capture and front capture) [note 2] that produce the "precursor water droplets" of the iberulites.[1][3][4] The movement of this water drop to lower tropospheric levels implies either simultaneous or consecutive processes such as coalescence, formation of vortex and downdraught. During this phase the iberulites acquire their spherical shape and internal structure (core and rind) although this shape can be distorted by adaptation to plant filaments.
Compositional attributes
The core is mainly formed by grains of quartz, calcite, dolomite and feldspars. The rind shows clay minerals, mainly smectites (beidellite, montmorillonite) and illite, sulfates, chlorides and amorphous silica. The latter group of minerals are mainly neoformed during the maturation process, that occurs in the atmosphere during the final stages of iberulite formation. Flight over areas with volcanic sulfur emissions (North Atlantic archipelagos) [note 3] probably incorporates SO2 onto the iberulite surface and descent to the marine boundary layer (MBL) [note 4] of the coast Iberian-Moroccan Atlantic leads to the incorporation of sea salt and microorganisms. Finally they fall on the southern Iberian Peninsula, where they were found.

Maturation
In detail, the atmospheric maturation process only occurs on the smectite rind, by means of heterogeneous and multiphase reactions [note 5] producing sulfates as the result of H2SO4 attack on the minerals of the rind. This would lead to the rapid transformation of some primary minerals into products of atmospheric neoformation [note 6] secondary minerals): the sulfates (mainly the gypsum) would be the product of H2SO4 attack on the interlayer cations of the smectites, which would gradually destroy the octahedral and tetrahedral [note 7] sheets of phyllosilicates creating mixed sulfates.
The alunite - jarosite found in the smectite rind would have a similar origin. If acid attack progresses further, the phyllosilicate grains would be completely destroyed, producing amorphous silica and releasing Fe. Since biogenic exoskeletons have no signs of corrosion, they must have been incorporated after the acid attack described above, probably simultaneously with the incorporation of sea salt.
Formation environment
Iberulites are linked to the evolution of high-dust air masses (plumes) which, originating in Saharan dust storms, are transported over the Iberian Peninsula and often across the eastern North Atlantic Ocean. These plumes occur in the warm season (May to September), as a result of anticyclonic activity affecting the Iberian Peninsula, and only sporadically in spring and autumn.
Notes (Associated concepts)
- ↑ Co-association: heterogeneous mixture of reactive mineral phases. These complex associations are typically formed in nature and are characterized by high surface area, low abundance of metal oxyhydroxide phases, and organic materials that act as cementing agents or surface coatings of prominent mineral grains.[2]
- ↑ Wake capture: This is a mode of aerodynamic capture of a drop falling in the atmosphere. A large drop settling through smaller drops will sweep out a volume and their hydrodynamic flow fields interfere collecting aerosols/droplets with some efficiency by the wake, depending on size of drops and size of aerosols, being most efficient for large and giant aerosols due to high terminal velocity and cross-sectional area.[4]
- ↑ Volcanic sulfur emissions: Sulfur and other gas emissions into the atmosphere from inside the earth occur near volcanic areas. These emissions can come from both clearly visible (explosive) eruptions and from diffuse (or quiescent) emissions, and there is no a real consensus about the relative importance of the latter. At present, submarine volcanic emissions occur in the mid-ocean ridges, and also as intraplate volcanism (hotspots); subaerial terrestrial volcanism is related with destructive plate margins Convergent boundary, Plate tectonics, (volcanic arcs above subduction zones).
- ↑ Marine boundary layer: This is defined as that part of the troposphere directly influenced by the presence of the ocean's surface. It reacts with little diurnal variability, is 1–2 km thick (3 km max), has a low Bowen ratio and a significant wave state. The marine boundary layer (MBL) over the earth's oceans plays a critical role in regulating surface energy and moisture fluxes and in controlling the convective transfer of energy and moisture to the free atmosphere.[5]
- ↑ Multiphase reactions: These refer to reactions involving components in different phases, and are a combination of simultaneous phase change and conversion of some materials into others. A general multiphase reaction generates three classes of fluxes: component mass sources, interphase mass transfer, interphase energy transfer.
- ↑ Neoformation: This is the formation of new mineral species from previously existing ones through alteration of environmental conditions. The new minerals thus produced are therefore stable in the new conditions.
- ↑ Tetrahedral, octahedral and interlayer sheets: The basic structural feature of the phyllosilicates is the stack of three types of layers: the tetrahedral sheet is formed by SiO4 tetrahedra, and each tetrahedron shares three of its vertex oxygen atoms with other tetrahedra and in which the Al can substitute up to half the Si. The octahedral sheet is made up by the Al, Fe and Mg cations, in six-coordination with the O and OH anions. Depending on the composition of the tetrahedral and octahedral sheets, the layer will have no charge, or will have a net negative charge. If the layers are charged this charge is balanced by interlayer cations such as Na+ or K+. In each case the interlayer can also contain water. The crystal structure is formed from a stack of layers interspaced with the interlayers.
See also
References
- 1 2 3 Díaz-Hernández, J.L. (2000). Aportaciones sólidas a la atmósfera originadas por un incendio forestal en el ámbito mediterráneo. Estudios Geológicos, 56: 153–161
- ↑ Berstch P. M. y Seaman J. C. (1999). «Characterization of complex mineral assemblages: implications for contaminant transport and environmental remediation». Proceedings National Academy of Sciences USA, 96: 3350–3357
- 1 2 Díaz-Hernández, J.L. y Párraga (2008) «The nature and tropospheric formation of iberulites: Pinkish mineral microspherulites». Geochimica et Cosmochimica Acta, 72: 3883–3906
- 1 2 3 Pruppacher H. R. y Klett J. D. (1997). Microphysics of clouds and precipitation (2ª ed.) Dordrecht: Kluwer Academic Publishers. 954 págs.
- ↑ Kloesel, K. A. y Albrecht, B. A. (1989). «Low-level inversions over the tropical Pacific. Thermodynamic structure of the boundary layer and the above inversion moisture structure». Monthly Weather Review, 117: 87-101