Joule heating
Joule heating, also known as ohmic heating and resistive heating, is the process by which the passage of an electric current through a conductor produces heat.
Joule's first law, also known as the Joule–Lenz law,[1] states that the power of heating generated by an electrical conductor is proportional to the product of its resistance and the square of the current:
Joule heating affects the whole electric conductor, unlike the Peltier effect which transfers heat from one electrical junction to another.
History
James Prescott Joule first published in December 1840, an abstract in the Proceedings of the Royal Society, suggesting that heat could be generated by an electrical current. Joule immersed a length of wire in a fixed mass of water and measured the temperature rise due to a known current flowing through the wire for a 30 minute period. By varying the current and the length of the wire he deduced that the heat produced was proportional to the square of the current multiplied by the electrical resistance of the immersed wire.[2]
In 1841 and 1842, subsequent experiments showed that the amount of heat generated was proportional to the chemical energy used in the voltaic pile that generated the current. This led Joule to reject the caloric theory (at that time the dominant theory) in favor of the mechanical theory of heat (according to which heat is another form of energy).[2]
Resistive heating was independently studied by Heinrich Lenz in 1842.[1]
The SI unit of energy was subsequently named the joule and given the symbol J. The commonly known unit of power, the watt, is equivalent to one joule per second.
Microscopic description
Joule heating is caused by interactions between the moving particles that form the current (usually, but not always, electrons) and the atomic ions that make up the body of the conductor. Charged particles in an electric circuit are accelerated by an electric field and have electrostatic potential energy. When the charged particles collide with ions in the conductor, the particles are scattered and so their motion becomes random and therefore thermal, increasing the temperature of the system as they continue to move through the circuit. Some kinetic energy is lost in these collisions however the drift velocities of these particles is of the order of mm/h and so kinetic energy loss is negligible and almost all kinetic energy comes from thermal motion. For example, carbon nanotubes have been found to emit infrared, visible, and ultraviolet radiation and reach temperatures over 4000 K when exposed to microwaves. Although a "perfect" carbon nanotube is theoretically capable of ballistic conduction, defects in nanotubes result in Joule heating when exposed to microwave fields.[3]
Power loss and noise
Joule heating is referred to as ohmic heating or resistive heating because of its relationship to Ohm's Law. It forms the basis for the large number of practical applications involving electric heating. However, in applications where heating is an unwanted by-product of current use (e.g., load losses in electrical transformers) the diversion of energy is often referred to as resistive loss. The use of high voltages in electric power transmission systems is specifically designed to reduce such losses in cabling by operating with commensurately lower currents. The ring circuits, or ring mains, used in UK homes are another example, where power is delivered to outlets at lower currents, thus reducing Joule heating in the wires. Joule heating does not occur in superconducting materials, as these materials have zero electrical resistance in the superconducting state.
Resistors create electrical noise, called Johnson–Nyquist noise. There is an intimate relationship between Johnson–Nyquist noise and Joule heating, explained by the fluctuation-dissipation theorem.
Formulas
Direct current
The most general and fundamental formula for Joule heating is:
where
- P is the power (energy per unit time) converted from electrical energy to thermal energy,
- I is the current traveling through the resistor or other element,
- is the voltage drop across the element.
The explanation of this formula (P=VI) is:[4]
- (Energy dissipated per unit time) = (Energy dissipated per charge passing through resistor) × (Charge passing through resistor per unit time)
When Ohm's law is also applicable, the formula can be written in other equivalent forms:
where R is the resistance.
Alternating current
When current varies, as it does in AC circuits,
where t is time and P is the instantaneous power being converted from electrical energy to heat. Far more often, the average power is of more interest than the instantaneous power:
where "avg" denotes average (mean) over one or more cycles, and "rms" denotes root mean square.
These formulas are valid for an ideal resistor, with zero reactance. If the reactance is nonzero, the formulas are modified:
where is the phase difference between current and voltage, means real part, Z is the complex impedance, and Y* is the complex conjugate of the admittance (equal to 1/Z*).
For more details in the reactive case, see AC power∆0}
Differential Form
In plasma physics, the Joule heating often needs to be calculated at a particular location in space. The differential form of the Joule heating equation gives the power per unit volume.
Here, is the current density, and is the electric field. For a neutral plasma not in magnetic field and with a conductivity , and therefore
where is the resistivity. This directly resembles the "" term of the macroscopic form.
Reason for high-voltage transmission of electricity
In electric power transmission, high voltage is used to reduce Joule heating of the overhead power lines. The valuable electric energy is intended to be used by consumers, not for heating the power lines. Therefore, this Joule heating is referred to as a type of transmission loss.
A given quantity of electric power can be transmitted through a transmission line either at low voltage and high current, or with a higher voltage and lower current. Transformers can convert a high transmission voltage to a lower voltage for use by customer loads. Since the power lost in the wires is proportional to the conductor resistance and the square of the current, using low current at high voltage reduces the loss in the conductors due to Joule heating (or alternatively allows smaller conductors to be used for the same relative loss).
Applications
Joule-heating or resistive-heating is used in uncountable number of gadgets and industrial process. The part of the gadget, that converts electricity into heat through the process of resistive or Joule heating is called a heating element.
| Gallery: Different applications of Joule heating | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
There are many practical uses of Joule heating:
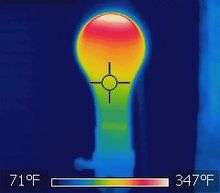
- An incandescent light bulb glows when the filament is heated by Joule heating, so hot that it glows white with thermal radiation (also called blackbody radiation).
- Electric stoves and other electric heaters usually work by Joule heating.
- Soldering irons and cartridge heaters are very often heated by Joule heating.
- Electric fuses rely on the fact that if enough current flows, enough heat will be generated to melt the fuse wire.
- Electronic cigarettes usually work by Joule heating, vaporizing propylene glycol and vegetable glycerine.
- Thermistors are resistors whose resistance changes when the temperature changes. These are sometimes used in conjunction with Joule heating: If a large current is sent through the thermistor, the device's temperature rises and therefore its resistance changes. If the device has a positive temperature coefficient of resistance (PTC), the rise in temperature will cause a drop in current, making the device useful in a circuit-protection role similar to fuses, or for feedback in circuits, or for many other purposes. In general, self-heating can turn a resistor into a nonlinear and hysteretic circuit element. For more details see thermistor#Self-heating effects.
- Food processing equipment may make use of Joule heating in food production. In this case, the food material serves as an electrical resistor, and heat is released internally.[5]
Heating efficiency
As a heating technology, Joule heating has a coefficient of performance of 1.0, meaning that every joule of electrical energy supplied produces one joule of heat. In contrast, a heat pump can have a coefficient of more than 1.0 since it moves additional thermal energy from the environment to the heated item.
The definition of the efficiency of a heating process requires defining the boundaries of the system to be considered. When heating a building, the overall efficiency is different when considering heating effect per unit of electric energy delivered on the customer's side of the meter, compared to the overall efficiency when also considering the losses in the power plant and transmission of power.
Hydraulic equivalent
In the energy balance of groundwater flow (see also Darcy's law) a hydraulic equivalent of Joule's law is used:[6]
where:
- = loss of hydraulic energy () due to friction of flow in -direction per unit of time (m/day) – comparable to
- = flow velocity in -direction (m/day) – comparable to
- = hydraulic conductivity of the soil (m/day) – the hydraulic conductivity is inversely proportional to the hydraulic resistance which compares to
See also
References
- 1 2 Джоуля — Ленца закон. Большая советская энциклопедия, 3-е изд., гл. ред. А. М. Прохоров. Москва: Советская энциклопедия, 1972. Т. 8 (A. M. Prokhorov; et al., eds. (1972). "Joule–Lenz law". Great Soviet Encyclopedia (in Russian). 8. Moscow: Soviet Encyclopedia.)
- 1 2 "This Month Physics History: December 1840: Joule's abstract on converting mechanical power into heat". aps.org. American Physical society. Retrieved 16 September 2016.
- ↑ Ferguson, S., Bhatnagar, P., Wright, I., Sestric, G., & Williams, S. (2015). Effects of Microwave Absorption on Long and Short Single-Walled Carbon Nanotubes at 10-6 Torr. International Journal of Nanoscience, 14(05n06), 1550025.
- ↑ Electric power systems: a conceptual introduction by Alexandra von Meier, p67, Google books link
- ↑ Ramaswamy, Raghupathy. "Ohmic Heating of Foods". Ohio State University. Retrieved 2013-04-22.
- ↑ R.J.Oosterbaan, J.Boonstra and K.V.G.K.Rao (1996). The energy balance of groundwater flow (PDF). In: V.P.Singh and B.Kumar (eds.), Subsurface-Water Hydrology, Vol.2 of the Proceedings of the International Conference on Hydrology and Water Resources, New Delhi, India. Kluwer Academic Publishers, Dordrecht, The Netherlands. pp. 153–160. ISBN 978-0-7923-3651-8.