Lawrencium
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol | lawrencium, Lr | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation |
lə-REN-see-əm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lawrencium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 103 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, block | group n/a, d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | actinide, sometimes considered a transition metal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight (Ar) | [266] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 7s2 7p1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
per shell | 2, 8, 18, 32, 32, 8, 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid (predicted) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1900 K (1627 °C, 2961 °F) (predicted) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density near r.t. | ~15.6–16.6 g/cm3 (predicted)[2][3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
1st: 478.6 kJ/mol[4] 2nd: 1428.0 kJ/mol (predicted) 3rd: 2219.1 kJ/mol (predicted) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure |
hexagonal close-packed (hcp)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 22537-19-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Ernest Lawrence | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Lawrence Berkeley National Laboratory and Joint Institute for Nuclear Research (1961–1971) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes of lawrencium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lawrencium is a synthetic chemical element with chemical symbol Lr (formerly Lw) and atomic number 103. It is named in honor of Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radioactive metal, lawrencium is the eleventh transuranic element and is also the final member of the actinide series. Like all elements with atomic number over 100, lawrencium can only be produced in particle accelerators by bombarding lighter elements with charged particles. Twelve isotopes of lawrencium are currently known; the most stable is 266Lr with a half-life of 11 hours, but the shorter-lived 260Lr (half-life 2.7 minutes) is most commonly used in chemistry because it can be produced on a larger scale.
Chemistry experiments have confirmed that lawrencium behaves as a heavier homolog to lutetium in the periodic table, and is a trivalent element. It thus could also be classified as the first of the 7th-period transition metals: however, its electron configuration is anomalous for its position in the periodic table, having an s2p configuration instead of the s2d configuration of its homolog lutetium. This means that lawrencium may be more volatile than expected for its position in the periodic table and have a volatility comparable to that of lead.
In the 1950s, 1960s, and 1970s, many claims of the synthesis of lawrencium of varying quality were made from laboratories in the Soviet Union and the United States. The priority of the discovery and therefore the naming of the element was disputed between Soviet and American scientists, and while the International Union of Pure and Applied Chemistry (IUPAC) established lawrencium as the official name for the element and gave the American team credit for the discovery, this was reevaluated in 1997, giving both teams shared credit for the discovery but not changing the element's name.
History

In 1958, scientists at the Lawrence Berkeley National Laboratory claimed the discovery of element 102, now called nobelium. At the same time, they also attempted to synthesize element 103 by bombarding the same curium target used with nitrogen-14 ions. A follow-up on this experiment was not performed, as the target was destroyed. Eighteen tracks were noted, with decay energy around (9 ± 1) MeV and half-life around 1⁄4 s; the Berkeley team noted that while the cause could be the production of an isotope of element 103, other possibilities could not be ruled out. While the data agrees reasonably with that later discovered for 257Lr (alpha decay energy 8.87 MeV, half-life 0.6 s), the evidence obtained in this experiment fell far short of the strength required to conclusively demonstrate the synthesis of element 103.[6][7] Later, in 1960, the Lawrence Berkeley Laboratory attempted to synthesize the element by bombarding 252Cf with 10B and 11B. The results of this experiment were not conclusive.[6]
The first important work on element 103 was carried out at Berkeley by the nuclear-physics team of Albert Ghiorso, Torbjørn Sikkeland, Almon Larsh, Robert M. Latimer, and their co-workers on February 14, 1961. The first atoms of lawrencium were reportedly produced by bombarding a three-milligram target consisting of three isotopes of the element californium with boron-10 and boron-11 nuclei from the Heavy Ion Linear Accelerator (HILAC).[8] The Berkeley team reported that the isotope 257103 was detected in this manner, and that it decayed by emitting an 8.6 MeV alpha particle with a half-life of (8 ± 2) s.[7] This identification was later corrected to be 258103,[8] as later work proved that 257Lr did not have the properties detected, but 258Lr did.[7] This was considered at the time to be convincing proof of the synthesis of element 103: while the mass assignment was less certain and proved to be mistaken, it did not affect the arguments in favor of element 103 having been synthesized. Scientists at the Joint Institute for Nuclear Research in Dubna (then in the Soviet Union) raised several criticisms: all but one were answered adequately. The exception was that 252Cf was the most common isotope in the target, and in the reactions with 10B, 258Lr could only have been produced by emitting four neutrons, and emitting three neutrons was expected to be much less likely than emitting four or five. This would lead to a narrow yield curve, not the broad one reported by the Berkeley team. A possible explanation was that there was a low number of events attributed to element 103.[7] This was an important intermediate step to the unquestioned discovery of element 103, although the evidence was not completely convincing.[7] The Berkeley team proposed the name "lawrencium" with symbol "Lw", after Ernest Orlando Lawrence, inventor of the cyclotron. The IUPAC Commission on Nomenclature of Inorganic Chemistry accepted the name, but changed the symbol to "Lr".[9] This acceptance of the discovery was later characterized as being hasty by the Dubna team.[7]
- 252
98Cf
+ 11
5B
→ 263
103Lr
* → 258
103Lr
+ 5 1
0
n
The first work at Dubna on element 103 came in 1965, when they reported to have created 256103 in 1965 by bombarding 243Am with 18O, identifying it indirectly from its granddaughter fermium-252. The half-life they reported was somewhat too high, possibly due to background events. Later 1967 work on the same reaction identified two decay energies in the ranges 8.35–8.50 MeV and 8.50–8.60 MeV: these were assigned to 256103 and 257103.[7] Despite repeated attempts, they were unable to confirm assignment of an alpha emitter with a half-life of eight seconds to 257103.[10][11] The Russians proposed the name "rutherfordium" for the new element in 1967:[6] this name was later used for element 104.
- 243
95Am
+ 18
8O
→ 261
103Lr
* → 256
103Lr
+ 5 1
0
n
Further experiments (Dubna 1969; Berkeley 1970) demonstrated an actinide chemistry for the new element, so by 1970 it was known that lawrencium is the last actinide.[7][12] In 1970, the Dubna group reported the synthesis of 255103 with half-life 20 s and alpha decay energy 8.38 MeV.[7] However, it was not until 1971, when the nuclear physics team at the University of California at Berkeley successfully performed a whole series of experiments aimed at measuring the nuclear decay properties of the lawrencium isotopes with mass numbers from 255 through 260,[13][14] that all previous results from Berkeley and Dubna were confirmed, apart from the Berkeley's group initial erroneous assignment of their first produced isotope to 257103 instead of the probably correct 258103.[7] All final doubts were finally dispelled in 1976 and 1977 when the energies of X-rays emitted from 258103 were measured.[7]

In 1971, the IUPAC granted the discovery of lawrencium to the Lawrence Berkeley Laboratory, even though they did not have ideal data for the element's existence. However, in 1992, the IUPAC Trans-fermium Working Group (TWG) officially recognized the nuclear physics teams at Dubna and Berkeley as the co-discoverers of lawrencium, concluding that while the 1961 Berkeley experiments were an important step to lawrencium's discovery, they were not yet completely convincing; and while the 1965, 1968, and 1970 Dubna experiments came very close to the needed level of confidence taken together, only the 1971 Berkeley experiments, which clarified and confirmed previous observations, finally resulted in complete confidence in the discovery of element 103.[6][9] Because the name "lawrencium" had been in use for a long time by this point, it was retained by IUPAC,[6] and in August 1997, the International Union of Pure and Applied Chemistry (IUPAC) ratified the name lawrencium and the symbol "Lr" during a meeting in Geneva.[9]
Characteristics
Physical
Lawrencium is the final member of the actinide series and is sometimes considered to be a group 3 element, along with scandium, yttrium, and lutetium, as its filled f-shell is expected to make it resemble the 7th-period transition metals. In the periodic table, it is located to the right of the actinide nobelium, to the left of the 6d transition metal rutherfordium, and under the lanthanide lutetium with which it shares many physical and chemical properties. Lawrencium is expected to be a solid under normal conditions and assume a hexagonal close-packed crystal structure (c/a = 1.58), similar to its lighter congener lutetium, though this is not yet known experimentally.[5] The enthalpy of sublimation of lawrencium is estimated to be 352 kJ·mol−1, close to the value of lutetium and strongly suggesting that metallic lawrencium is trivalent with the 7s and 6d electrons delocalized, a prediction also supported by a systematic extrapolation of the values of heat of vaporization, bulk modulus, and atomic volume of neighboring elements to lawrencium.[15] Specifically, lawrencium is expected to be a trivalent, silvery metal, easily oxidized by air, steam, and acids,[16] and having an atomic volume similar to that of lutetium and a trivalent metallic radius of 171 pm.[15] It is expected to be a rather heavy metal with a density of around 15.6 to 16.6 g·cm−3.[2][3] It is also predicted to have a melting point of around 1900 K (1627 °C), not far from the value for lutetium (1925 K).[17]
Chemical
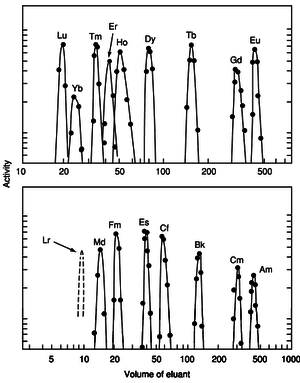
In 1949, Glenn T. Seaborg, who devised the actinide concept that elements 89 to 103 formed an actinide series homologous to the lanthanide series from elements 57 to 71, predicted that element 103 (lawrencium) should be its final member and that the Lr3+ ion should be about as stable as Lu3+ in aqueous solution. It was not until decades later that element 103 was finally conclusively synthesized and this prediction was experimentally confirmed.[18]
1969 studies on the element showed that lawrencium reacted with chlorine to form a product that was most likely the trichloride LrCl3. Its volatility was found to be similar to that of the chlorides of curium, fermium, and nobelium and much less than that of rutherfordium chloride. In 1970, chemical studies were performed on 1500 atoms of the isotope 256Lr, comparing it with divalent (No, Ba, Ra), trivalent (Fm, Cf, Cm, Am, Ac), and tetravalent (Th, Pu) elements. It was found that lawrencium coextracted with the trivalent ions, but the short half-life of the 256Lr isotope precluded a confirmation that it eluted ahead of Md3+ in the elution sequence.[18] Lawrencium occurs as the trivalent Lr3+ ion in aqueous solution and hence its compounds should be similar to those of the other trivalent actinides: for example, lawrencium(III) fluoride (LrF3) and hydroxide (Lr(OH)3) should both be insoluble in water.[18] Due to the actinide contraction, the ionic radius of Lr3+ should be smaller than that of Md3+, and that it should elute ahead of Md3+ when ammonium α-hydroxyisobutyrate (ammonium α-HIB) is used as an eluant.[18] Later 1987 experiments on the longer-lived isotope 260Lr confirmed lawrencium's trivalency and that it eluted in roughly the same place as erbium, and found that lawrencium's ionic radius was (88.6 ± 0.3) pm, larger than would be expected from simple extrapolation from periodic trends.[18] Later 1988 experiments with more lawrencium atoms refined this value to (88.1 ± 0.1) pm and calculated an enthalpy of hydration value of −(3685 ± 13) kJ·mol−1.[18] It was also pointed out that the actinide contraction at the end of the actinide series was larger than the analogous lanthanide contraction, with the exception of the last actinide, lawrencium: the cause was speculated to be relativistic effects.[18]
It has been speculated that the 7s electrons are relativistically stabilized, so that in reducing conditions, only the 7p1/2 or 6d electron would be ionized, leading to the monovalent Lr+ ion. However, all experiments to reduce Lr3+ to Lr2+ or Lr+ in aqueous solution were unsuccessful. On the basis of this, the standard electrode potential of the E°(Lr3+→Lr+) couple was calculated to be less than −1.56 V, indicating that the existence of Lr+ ions in aqueous solution was unlikely. The upper limit for the E°(Lr3+→Lr2+) couple was predicted to be −0.44 V: the values for E°(Lr3+→Lr) and E°(Lr4+→Lr3+) are predicted to be −2.06 V and +7.9 V.[18] The stability of the group oxidation state in the 6d transition series decreases as Rf4+ > Db5+ > Sg6+, and lawrencium continues the trend with Lr3+ being more stable than Rf4+.[19]
Atomic
A lawrencium atom has 103 electrons, of which three can act as valence electrons. In 1970, it was predicted that the ground-state electron configuration of lawrencium was [Rn]5f146d17s2 (ground state term symbol 2D3/2), following the Aufbau principle and conforming to the [Xe]4f145d16s2 configuration of lawrencium's lighter homolog lutetium.[20] However, the next year, calculations were published that questioned this prediction, instead expecting an anomalous [Rn]5f147s27p1 configuration.[20] Though early calculations gave conflicting results,[21] more recent studies and calculations confirm the s2p suggestion.[22][23] 1974 relativistic calculations concluded that the energy difference between the two configurations was small and that it was uncertain which was the ground state.[20] Later 1995 calculations concluded that the s2p configuration should be energetically favored, because the spherical s and p1/2 orbitals are nearest to the atomic nucleus and thus move quickly enough that their relativistic mass increases significantly.[20]
In 1988, a team of scientists led by Eichler calculated that lawrencium's enthalpy of adsorption on metal sources would differ enough depending on its electron configuration that it would be feasible to carry out experiments to exploit this fact to measure lawrencium's electron configuration.[20] The s2p configuration was expected to be more volatile than the s2d configuration, and be more similar to that of the p-block element lead. No evidence for lawrencium being volatile was obtained and the lower limit for the enthalpy of adsorption of lawrencium on quartz or platinum was significantly higher than the estimated value for the s2p configuration.[20]
In 2015, the first ionization energy of lawrencium was measured, using the isotope 256Lr.[24] The measured value, 4.96+0.08
−0.07 eV, agreed very well with the relativistic theoretical prediction of 4.963(15) eV, and also provided a first step into measuring the first ionization energies of the transactinides.[24] This value is the lowest among all the lanthanides and actinides, and supports the s2p configuration as the 7p1/2 electron is expected to be only weakly bound. This suggests that lutetium and lawrencium behave similarly to the d-block elements (and hence being the true heavier congeners of scandium and yttrium, instead of lanthanum and actinium), and also that lawrencium may behave similarly to the alkali metals sodium and potassium in some ways.[25] Given that the s2p configuration is correct, then lawrencium cannot be regarded as a transition metal under the IUPAC definition ("An element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell"),[26] unlike its lighter homolog lutetium and the group 3 elements, with which lutetium and lawrencium are sometimes classified.[27] It is nevertheless quite likely that metallic lawrencium will behave similarly to curium and show the expected [Rn]5f146d17s2 configuration, which is supported by the earlier volatility experiments.[28]
Isotopes
Twelve isotopes of lawrencium are known, with mass numbers 252–262 and 266; all are radioactive.[29][30] Additionally, one nuclear isomer is known, with mass number 253.[29] The longest-lived lawrencium isotope, 266Lr, has a half-life of 11 hours and is one of the longest lived superheavy isotopes known to date, suggesting that it is perhaps on the shore of the island of stability of superheavy nuclei.[31] However, shorter-lived isotopes are usually used in chemical experiments because 266Lr currently can only be produced as a final decay product of even heavier and harder-to-synthesize elements: it was discovered in 2014 in the decay chain of tennessine-294.[29][30] The isotope 256Lr (half-life 27 seconds) was used in the first chemical studies on lawrencium: currently, the slightly longer lived isotope 260Lr (half-life 2.7 minutes) is usually used for this purpose.[29] After 266Lr, the longest-lived lawrencium isotopes are 262Lr (3.6 h), 261Lr (44 min), 260Lr (2.7 min), 256Lr (27 s), and 255Lr (22 s).[29][32][33] All other known lawrencium isotopes have half-lives under 20 seconds, and the shortest-lived of them (252Lr) has a half-life of only 390 milliseconds.[29][32][33] However, the undiscovered isotopes with mass numbers 263 to 265 are expected to have longer half-lives (263Lr, 5 h; 264Lr and 265Lr, 10 h).[32][33] The half-lives of lawrencium isotopes mostly increase smoothly from 252Lr to 266Lr, with a dip from 257Lr to 259Lr.[29][32][33]
Preparation and purification
While the lightest (252Lr to 254Lr) and heaviest (266Lr) lawrencium isotopes are produced only as alpha decay products of dubnium (Z = 105) isotopes, the middle isotopes (255Lr to 262Lr) can all be produced by bombarding actinide (americium to einsteinium) targets with light ions (from boron to neon). The two most important isotopes, 256Lr and 260Lr, are both in this range. 256Lr can be produced by bombarding californium-249 with 70 MeV boron-11 ions (producing lawrencium-256 and four neutrons), while 260Lr can be produced by bombarding berkelium-249 with oxygen-18 (producing lawrencium-260, an alpha particle, and three neutrons).[34]
Both 256Lr and 260Lr have half-lives too short to allow a complete chemical purification process. Early experiments with 256Lr therefore used rapid solvent extraction, with the chelating agent thenoyltrifluoroacetone (TTA) dissolved in methyl isobutyl ketone (MIBK) as the organic phase, and with the aqueous phase being buffered acetate solutions. Ions of different charge (+2, +3, or +4) will then extract into the organic phase under different pH ranges, but this method will not separate the trivalent actinides and thus 256Lr must be identified by its emitted 8.24 MeV alpha particles.[34] More recent methods have allowed rapid selective elution with α-HIB to take place in enough time to separate out the longer-lived isotope 260Lr, which can be removed from the catcher foil with 0.05 M hydrochloric acid.[34]
References
- ↑ Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. p. 278–9. ISBN 978-0-19-960563-7.
- 1 2 Fournier, Jean-Marc (1976). "Bonding and the electronic structure of the actinide metals". Journal of Physics and Chemistry of Solids. 37 (2): 235–244. doi:10.1016/0022-3697(76)90167-0.
- 1 2 Penneman, R. A.; Mann, J. B. (1976). "'Calculation chemistry' of the superheavy elements; comparison with elements of the 7th period". Proceedings of the Moscow Symposium on the Chemistry of Transuranium Elements: 257–263. doi:10.1016/B978-0-08-020638-7.50053-1.
- ↑ http://cen.acs.org/articles/93/i15/Lawrencium-Ionization-Energy-Measured.html?cq_ck=1428631698138
- 1 2 Östlin, A.; Vitos, L. (2011). "First-principles calculation of the structural stability of 6d transition metals". Physical Review B. 84 (11). Bibcode:2011PhRvB..84k3104O. doi:10.1103/PhysRevB.84.113104.
- 1 2 3 4 5 Emsley, John (2011). Nature's Building Blocks.
- 1 2 3 4 5 6 7 8 9 10 11 Barber, R. C.; Greenwood, N.N.; Hrynkiewicz, A.Z.; Jeannin, Y.P.; Lefort, M.; Sakai, M.; Ulehla, I.; Wapstra, A.P.; Wilkinson, D.H. (1993). "Discovery of the transfermium elements. Part II: Introduction to discovery profiles. Part III: Discovery profiles of the transfermium elements". Pure and Applied Chemistry. 65 (8): 1757. doi:10.1351/pac199365081757. (Note: for Part I see Pure Appl. Chem., Vol. 63, No. 6, pp. 879–886, 1991)
- 1 2 Ghiorso, Albert; Sikkeland, T.; Larsh, A. E.; Latimer, R. M. (1961). "New Element, Lawrencium, Atomic Number 103". Phys. Rev. Lett. 6 (9): 473. Bibcode:1961PhRvL...6..473G. doi:10.1103/PhysRevLett.6.473.
- 1 2 3 Greenwood, Norman N. (1997). "Recent developments concerning the discovery of elements 101–111". Pure & Appl. Chem. 69 (1): 179–184. doi:10.1351/pac199769010179.
- ↑ Flerov, G. N. (1967). "On the nuclear properties of the isotopes 256103 and 257103". Nucl. Phys. A. 106: 476. Bibcode:1967NuPhA.106..476F. doi:10.1016/0375-9474(67)90892-5.
- ↑ Donets, E. D.; Shchegolev, V. A.; Ermakov, V. A. (1965). Atomnaya Énergiya (in Russian). 19 (2): 109. Missing or empty
|title=(help)
- Translated in Donets, E. D.; Shchegolev, V. A.; Ermakov, V. A. (1965). "Synthesis of the isotope of element 103 (lawrencium) with mass number 256". Soviet Atomic Energy. 19 (2): 109. doi:10.1007/BF01126414.
- ↑ Kaldor, Uzi & Wilson, Stephen (2005). Theoretical chemistry and physics of heavy and superheavy element. Springer. p. 57. ISBN 1-4020-1371-X.
- ↑ Silva, pp. 1641–2
- ↑ Eskola, Kari; Eskola, Pirkko; Nurmia, Matti; Albert Ghiorso (1971). "Studies of Lawrencium Isotopes with Mass Numbers 255 Through 260". Phys. Rev. C. 4 (2): 632–642. Bibcode:1971PhRvC...4..632E. doi:10.1103/PhysRevC.4.632.
- 1 2 Silva, p. 1644
- ↑ John Emsley (2011). Nature's Building Blocks: An A-Z Guide to the Elements. Oxford University Press. pp. 278–9. ISBN 978-0-19-960563-7.
- ↑ Lide, D. R., ed. (2003). CRC Handbook of Chemistry and Physics (84th ed.). Boca Raton, FL: CRC Press.
- 1 2 3 4 5 6 7 8 Silva, pp. 1644–7
- ↑ Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. p. 1686. ISBN 1-4020-3555-1.
- 1 2 3 4 5 6 Silva, pp. 1643–4
- ↑ Nugent, L.J.; Vander Sluis, K.L.; Fricke, Burhard; Mann, J.B. (1974). "Electronic configuration in the ground state of atomic lawrencium" (PDF). Phys. Rev. A. 9 (6): 2270–72. Bibcode:1974PhRvA...9.2270N. doi:10.1103/PhysRevA.9.2270.
- ↑ Eliav, E.; Kaldor, U.; Ishikawa, Y. (1995). "Transition energies of ytterbium, lutetium, and lawrencium by the relativistic coupled-cluster method". Phys. Rev. A. 52: 291–296. Bibcode:1995PhRvA..52..291E. doi:10.1103/PhysRevA.52.291.
- ↑ Zou, Yu; Froese Fischer C.; Uiterwaal, C.; Wanner, J.; Kompa, K.-L. (2002). "Resonance Transition Energies and Oscillator Strengths in Lutetium and Lawrencium". Phys. Rev. Lett. 88 (2): 183001. Bibcode:2002PhRvL..88b3001M. doi:10.1103/PhysRevLett.88.023001. PMID 12005680.
- 1 2 Sato, T. K.; Asai, M.; Borschevsky, A.; Stora, T.; Sato, N.; Kaneya, Y.; Tsukada, K.; Düllman, Ch. E.; Eberhardt, K.; Eliav, E.; Ichikawa, S.; Kaldor, U.; Kratz, J. V.; Miyashita, S.; Nagame, Y.; Ooe, K.; Osa, A.; Renisch, D.; Runke, J.; Schädel, M.; Thörle-Pospiech, P.; Toyoshima, A.; Trautmann, N. (9 April 2015). "Measurement of the first ionization potential of lawrencium, element 103". Nature. 520: 209–11. Bibcode:2015Natur.520..209S. doi:10.1038/nature14342.
- ↑ Gunther, Matthew (9 April 2015). "Lawrencium experiment could shake up periodic table". RSC Chemistry World. Retrieved 21 September 2015.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "transition element".
- ↑ "WebElements Periodic Table of the Elements". Webelements.com. Retrieved 2010-04-03.
- ↑ Haire, R. G. (11 October 2007). "Insights into the bonding and electronic nature of heavy element materials". Journal of Alloys and Compounds. 444–5: 63–71. doi:10.1016/j.jallcom.2007.01.103.
- 1 2 3 4 5 6 7 Silva, p. 1642
- 1 2 Khuyagbaatar, J.; et al. (2014). "48Ca + 249Bk Fusion Reaction Leading to Element Z = 117: Long-Lived α-Decaying 270Db and Discovery of 266Lr". Physical Review Letters. 112 (17). Bibcode:2014PhRvL.112q2501K. doi:10.1103/PhysRevLett.112.172501.
- ↑ Clara Moskowitz (May 7, 2014). "Superheavy Element 117 Points to Fabled "Island of Stability" on Periodic Table". Scientific American. Retrieved 2014-05-08.
- 1 2 3 4 http://www.nucleonica.net/unc.aspx
- 1 2 3 4 Audi, G.; Bersillon, O.; Blachot, J.; Wapstra, A. H. (2003), "The NUBASE evaluation of nuclear and decay properties" (PDF), Nucl. Phys. A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- 1 2 3 Silva, pp. 1642–3
Bibliography
- Silva, Robert J. (2011). "Chapter 13. Fermium, Mendelevium, Nobelium, and Lawrencium". In Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements. Netherlands: Springer. doi:10.1007/978-94-007-0211-0_13. ISBN 978-94-007-0210-3.
External links
- "Chart of Nuclides". National Nuclear Data Center (NNDC). Retrieved 2014-08-21.
- Los Alamos National Laboratory's Chemistry Division: Periodic Table – Lawrencium
- Lawrencium at The Periodic Table of Videos (University of Nottingham)
| Periodic table (Large cells) | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||||
| 1 | H | He | |||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
|
| |||||||||||||||||||||||||||||||||