Lek mating
.jpg)
A lek is an aggregation of males gathered to engage in competitive displays, lekking, that may entice visiting females who are surveying prospective partners for copulation.[1] Leks are commonly formed before or during the breeding season. A lekking species is characterised by male displays, strong female mate choice, and the conferring of male indirect benefits.[2] Although lekking is most prevalent among avian species, lekking behavior is found in insects, amphibians, and mammals as well.
Etymology
The term derives from the Swedish lek, a noun which typically denotes pleasurable and less rule-bound games and activities ("play", as by children). English use of lek dates to the 1860s. Llewelyn Lloyd's The Game birds and wild fowl of Sweden and Norway (1867) introduces it (capitalised and in single quotes, as 'Lek') explicitly as a Swedish term.[5]
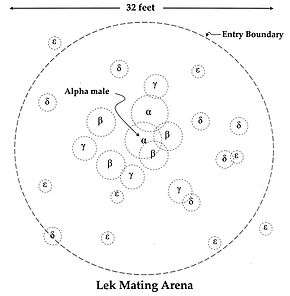
Lekking species
The term was originally used most commonly for black grouse (Swedish: "orrlek") and for capercaillie (Swedish: "tjäderlek"), and lekking behavior is quite common in birds of this type, such as sage grouse, prairie chicken, great bustard and sharp-tailed grouse. However, lekking is also found in birds of other families, such as the ruff, great snipe, Guianan cock-of-the-rock, musk ducks, hermit hummingbirds, manakins, birds-of-paradise, screaming pihas and the kakapo. Lekking is seen in some mammals such as the Ugandan kob (a waterbuck), some pinnipeds, several species of fruit bat, and the topi antelope. Lekking is found in marine iguanas[6] and some species of fish (e.g., Atlantic cod, desert pupfish,[7] and the cichlid Astatotilapia burtoni). Even insects like the midge and the ghost moth demonstrate lekking behavior. Lekking is also found in some paper wasp species such as Polistes dominula,[8] the orchid bee Eulaema meriana, [9] in some butterfly species like the black swallowtail (Papilio polyxenes),[10] and in tarantula hawks like Hemipepsis ustulata.
Lekking behaviour

There are two types of lekking arrangements: classical and exploded. In the classic lekking system, male territories are in visual and auditory range of their neighbours. In an exploded lek, males are further away from one another than they would be in a classic lek. Males in an exploded lek are outside of visual range of one another, but they stay within earshot.[11] Exploded lek territories are much more expansive than classic systems and are known to exhibit more variation.[12] A famous example of exploded leks is the "booming" call of the kakapo, the males of which position themselves many kilometres apart from one another to signal to potential mates.[13]
Lek territories of different taxa are stable and do not vary in terms of size and location.[14] Males often return to the same mating sites because of female fidelity.[15] It has been shown that avian females such as the black grouse and great snipe are faithful to males and not mating sites.[16] Successful males congregate in the same area as the previous breeding season because it is familiar to them, while females return to reunite with said males. It has been observed that they do not return to a mating site if their male partner is not present.[16] Another possible explanation for lek stability may result from male hierarchies within a lek. In manakins, subordinate betas may inherit an alpha’s display site, increasing the chances of female visitation.[16] Rank may also contribute to the stability of lek size, as lower ranking males may congregate to achieve a perceived optimal size as a way to attract females.[17]
Some species of ants, such as red harvester ants, as well as certain bee species, like Tetragonisca angustula and Trigona spinipes exhibit lek-like mating patterns.[18][19] Males form reproductive aggregations, congregating and collectively give off a pheromone that attracts reproductive females. The more males present to give off the pheromone, the stronger the attraction for the females.
Lek mating is relatively common among paper wasp species. For example, Polistes dominula males often fight with other males in mid-air to demonstrate their superiority and attractiveness. Males that lose fly away from the lek. Females fly through leks or perch near lekking areas to observe males before making choices on mates and they use the highly conspicuous abdominal spots on males, which are highly variable in size and shape, to aid in mate choice. Males with smaller, more elliptically shaped spots are more dominant over other males and preferred by females compared to males who have larger, more irregularly shaped spots.[20] In comparison, Mischocyttarus flavitarsis males choose a perch site near female hibernation areas, rub their abdomens to mark their territory and wait 6–7 weeks for a female to approach. If an intruder approaches, the owner of the site lunges and grapples the other wasp. Typically, they fall off the perch site and finish the fight on the ground.[21]
Costs and benefits

The main benefit for both sexes is mating success. For males, the costs stem from females’ preferences. The traits that are selected for may be energetically costly to maintain and may cause increased predation. For example, increased vocalization rate caused a decrease in the mass of male great snipe.[22] Another cost would be male competition, as females prefer victorious males. Great snipes regularly fight to display dominance or defend their territory.[22] Aggressive male black grouse are preferred over non-aggressive males and when the males fight they tear feathers from each other's tails.[23] At first glance, it would seem that females receive no direct benefits because these males are only contributing genes to the offspring.[24] However, lekking actually reduces the cost of female searching because the congregating of males makes mate selection easier.[25] Females do not have to travel as far, since they are able to evaluate and compare multiple males within the same vicinity. This may also help reduce the amount of time a female may be vulnerable to predators. Female Hyperolius marmoratus under predatory pressure consistently chose leks near their release sites, and high male calling rates reduced female search time.[26]
Female mating preferences
A meta analysis of 27 species found that qualities such as lekking size, male display rate, and the rate of male aggression exhibit positive correlation with male success rates.[1] A positive correlation was also found between attendance, magnitude of exaggerated traits, age, frequency of fights, and mating success.[1] This female preference leads to mating skew, with some males being more successful at copulating with females. The variation in mating success is quite large in lek mating systems with 70-80 percent of matings being attributed to only 10%-20% of the males present.[27]
The lek paradox

The conundrum of how additive or beneficial genetic variation is maintained in the face of the consistent female preferences is known as the “lek paradox." While many studies have attempted to explain how the lek paradox fits into Darwinian theory, the paradox remains. Persistent female choice for particular male trait values should erode genetic diversity in male traits and thereby remove the benefits of choice, yet choice persists.[28] This paradox can be somewhat alleviated by the occurrence of mutations introducing potential differences, as well as the possibility that traits of interest have more or less favorable recessive alleles.
The basis of the lek paradox is continuous genetic variation in spite of strong female preference for certain traits. There are two conditions in which the lek paradox arises. The first is that males contribute only genes and the second is that female preference does not affect fecundity.[29] Female choice should lead to directional runaway selection, resulting in a greater prevalence for the selected traits. Stronger selection should lead to impaired survival, as it decreases genetic variance and ensures that more offspring have similar traits.[30] However, lekking species do not exhibit runaway selection.
In a lekking reproductive system, what male sexual characteristics can signal to females is limited, as the males provide no resources to females or parental care to their offspring.[31] This implies that females gain indirect benefits from her choice in the form of “good genes” for her offspring.[32] Hypothetically, in choosing a male that excels at courtship displays, females gain genes for their offspring that increase their survival or reproductive fitness.
Amotz Zahavi declared that male sexual characteristics only convey useful information to the females if these traits confer a handicap on the male.[33] Otherwise, males could simply cheat: if the courtship displays have a neutral effect on survival, males could all perform equally and it would signify nothing to the females. But if the courtship display is somehow deleterious to the male’s survival—such as increased predator risk or time and energy expenditure—it becomes a test by which females can assess male quality. Under the handicap principle, males who excel at the courtship displays prove that they are of better quality and genotype, as they have already withstood the costs to having these traits.[33] Resolutions have been formed to explain why strong female mate choice does not lead to runaway selection. The handicap principle describes how costly male ornaments provide females with information about the male’s inheritable fitness.[34] The handicap principle may be a resolution to the lek paradox, for if females select for the condition of male ornaments, then their offspring have better fitness.
One potential resolution to the lek paradox is Rowe and Houle's theory of condition-dependent expression of male sexually selected traits. Similar to the handicap principle, Rowe and Houle argue that sexually selected traits depend on physical condition. Condition, in turn, summarizes a large number of genetic loci, including those involved in metabolism, muscular mass, nutrition, etc. Rowe and Houle claim that condition dependence maintains genetic variation in the face of persistent female choice, as the male trait is correlated with abundant genetic variation in condition.[32] This is the genic capture hypothesis, which describes how a significant amount of the genome is involved in shaping the traits that are sexually selected.[31] There are two criteria in the genic capture hypothesis: the first is that sexually selected traits are dependent upon condition and the second is that general condition is attributable to high genetic variance.[35]
Genetic variation in condition-dependent traits may be further maintained through mutations and environmental effects. Genotypes may be more effective in developing condition dependent sexual characteristics in different environments, while mutations may be deleterious in one environment and advantageous in another.[31] Thus genetic variance remains in populations through gene flow across environments or generation overlap. According to the genic capture hypothesis, female selection does not deplete the genetic variance, as sexual selection operates on condition dependence traits, thereby accumulating genetic variance within the selected for trait.[35] Therefore, females are actually selecting for high genetic variance.
In an alternate but non-exclusionary hypothesis, W. D. Hamilton and M. Zuk proposed that successful development of sexually selected traits signal resistance to parasites.[36] Parasites can significantly stress their hosts so that they are unable to develop sexually selected traits as well as healthy males. According to this theory, a male who vigorously displays demonstrates that he has parasite-resistant genes to the females. In support of this theory, Hamilton and Zuk found that male sexual ornaments were significantly correlated with levels of incidence of six blood diseases in North American passerine bird species. The Hamilton and Zuk model addresses the lek paradox, arguing that the cycles of co-adaptation between host and parasite resist a stable equilibrium point. Hosts continue to evolve resistance to parasites and parasites continue to bypass resistant mechanisms, continuously generating genetic variation.[36] The genic capture and parasite resistance hypotheses could logically co-occur in the same population.
One resolution to the lek paradox involves female preferences and how preference alone does not cause a drastic enough directional selection to diminish the genetic variance in fitness.[37] Another conclusion is that the preferred trait is not naturally selected for or against and the trait is maintained because it implies increased attractiveness to the male.[29] Thus, there may be no paradox.
Evolution
Hotshot hypothesis
There have been several hypotheses proposed as to why males cluster into leks. The hotshot hypothesis is the only model that attributes males as the driving force behind aggregation. The hotshot model hypothesizes that attractive males, known as hotshots, garner both female and male attention.[38] Females go to the hotshots because they are attracted to these males. Other males form leks around these hotshots as a way to lure females away from the hotshot. A manipulative experiment using the little bustard, Tetrax tetrax, was done to test the various lek evolution models.[39] The experiment involved varying the size and sex ratio of leks using decoys. To test whether or not the presence of a hotshot determined lek formation, a hotshot little bustard decoy was placed within a lek. After the fake hotshot was added to the lek, both male and female visitation to the lek increased.[39]
Hotspot model
The hotspot model considers the female density to be the catalyst for the clustering of males. This model predicts that leks will form where females tend to reside as a way to increase female interaction.[40] Female manakin traffic has been observed to be concentrated around leks, bathing sites, and fruiting areas, with males aggregated near the most visited fruiting resources.[40] The hotspot model also predicts that lek size is dependent upon the number of females inhabiting a patch of land.[38] To test if the number of females affects lek formation, a group of female little bustard decoys were added to a lek. The presence of these female decoys did not have an effect on lek size.[39]
Blackhole model
The blackhole model proposes that females have a preference for neither size nor type of male, but rather that females tend to be mobile and mate wherever leks may be located.[39] This model predicts that female mobility is a response to male harassment.[41] This prediction is difficult to test, but there was a negative correlation found between male aggressiveness and female visitation in the little bustard population.[39] Evidence supporting the black hole model is mainly found in ungulates.[15]
Kin selection
An alternative hypothesis for lekking is kin selection, which assumes that males within a lek are related to one another. As females rarely mate outside of leks, it is advantageous for males to form leks.[42] Although not all males within a lek mate with a female, the unmated males still receive fitness benefits. Kin selection explains that related males congregate to form leks, as a way to attract females and increase inclusive fitness.[16] In some species, the males at the leks show a high degree of relatedness, but this does not apply as a rule to lek-forming species in general.[43][44][45] In a few species such as peacocks and the black grouse, leks are composed of brothers and half-brothers. The lower-ranking males gain some fitness benefit by passing their genes on through attracting mates for their brothers (larger leks attract more females). Peacocks recognize and will lek with their brothers, even if they have never met before.[46]
Predation protection
Another hypothesis is predator protection, or the idea that there is a reduction in individual predation risk in a larger group.[39] This could work both for the males in within the group as well as any female who visits the lek.[47] Protection also explains the presence of mixed leks, when a male of one species joins a lek of another species for protection from a common set of predators. This occurs with manakins,[48] as well as other birds such as grouse species.[49]
References
- 1 2 3 Fiske, P.; Rintamaki, P. T.; Karvonen, E. (1998). "Mating success in lekking males: a meta-analysis". Behavioral Ecology. 9: 328–338. doi:10.1093/beheco/9.4.328.
- ↑ Frédéric Jiguet; Beatriz Arroyo; Vincent Bretagnolle (October 2000). "Lek Mating Systems: A Case Study in the Little Bustard Tetrax tetrax". Behavioural Processes. 51 (1–3): 63–82. doi:10.1016/S0376-6357(00)00119-4.
- ↑ Starr, Cecie; Taggart, Ralph (1992). Biology: The Unity and Diversity of Life (6th ed.). Wadsworth. ISBN 0-534-16566-4.
- ↑ Hall, Edward T. (1966). The Hidden Dimension. Anchor Books. ISBN 0-385-08476-5.
- ↑ Lloyd, Llewelyn (1867). The Game Birds and Wild Fowl of Sweden and Norway. London: Frederick Warne & Co. pp. 219ff.url=https://books.google.com/books?id=SEd–EgZE2AgC.. Lloyd also loans 'Lek-ställe' (Swedish lekställe) for "pairing ground".
- ↑ Vitousek, Maren N.; Mitchell, Mark A.; Woakes, Anthony J.; Niemack, Michael D.; Wikelski, Martin; Tregenza, Tom (2007). "High Costs of Female Choice in a Lekking Lizard". PLoS ONE. 2 (6): e567. Bibcode:2007PLoSO...2..567V. doi:10.1371/journal.pone.0000567. PMC 1891434
 . PMID 17593966.
. PMID 17593966. 
- ↑ Loiselle, Paul V. (December 1982). "Male Spawning-Partner Preference in an Arena-Breeding Teleost Cyprinodon macularius californiensis Girard (Atherinomorpha: Cyprinodontidae)". The American Naturalist. 120 (6): 721–732. doi:10.1086/284026.
- ↑ Cappa, F.; Bruschini, C.; Cervo, R.; Turillazzi, S.; Beani, L. (2013). "Males do not like the working class: male sexual preference and recognition of functional castes in a primitively eusocial wasp". Animal Behaviour. 86 (4): 801–810. doi:10.1016/j.anbehav.2013.07.020.
- ↑ Kimsey, Lynn Siri (1980-11-01). "The behaviour of male orchid bees (Apidae, Hymenoptera, Insecta) and the question of leks". Animal Behaviour. 28 (4): 996–1004. doi:10.1016/S0003-3472(80)80088-1.
- ↑ Lederhouse, Robert C (1982). "Territorial Defense and Lek Behavior of the Black Swallowtail Butterfly, Papilio polyxenes". Behavioral Ecology and Sociobiology. 10 (2): 109–118. doi:10.1007/bf00300170. JSTOR 4599468.
- ↑ Trail, P. W. (1990). "Why Should Lek-Breeders be Monomorphic?". Evolution. 44: 1837–1852. doi:10.2307/2409512.
- ↑ Jiguet, F.; Arroyo, B.; Bretagnolle, V. (2000). "Lek mating systems: a case study in the Little Bustard Tetrax tetrax". Behavioural Processes. 51: 63–82. doi:10.1016/s0376-6357(00)00119-4.
- ↑ Merton, Don V; Morris, Rodney B.; Atkinson, Ian A.E. (1984). "Lek behaviour in a parrot: The Kakapo Strigops habroptilus of New Zealand". Ibis. 126 (3): 277–283. doi:10.1111/j.1474-919X.1984.tb00250.x.
- ↑ Durães, R.; Loiselle; Parker, P. G.; Blake, J. G. (2009). "Female mate choice across spatial scales: influence of lek and male attributes on mating success of blue-crowned manakins". Proceedings of the Royal Society B: Biological Sciences. 276: 1875–1881. doi:10.1098/rspb.2008.1752. PMC 2674486
 . PMID 19324796.
. PMID 19324796. - 1 2 Isvaran, K (2005). "Variation in male mating behaviour within ungulate populations: patterns and processes". Current Science. 89 (7): 1192–1199.
- 1 2 3 4 Duval, E. H. (2013). "Female mate fidelity in a lek mating system and its implications for the evolution of cooperative lekking behavior". The American Naturalist. 181: 213–22. doi:10.1086/668830.
- ↑ Hernandez, M. L.; Houston, A. I.; Mcnamara, J. M. (1999). "Male rank and optimal lek size". Behavioral Ecology. 10: 73–79. doi:10.1093/beheco/10.1.73.
- ↑ Velthuis, Hayo H.W.; Koedam, Dirk; Imperatriz-Fonseca, Vera L (2005). "The males of Melipona and other stingless bees, and their mothers". Apidologie. 36 (2): 169–185. doi:10.1051/apido:2005014.
- ↑ Prato, M; Soares, A E E (July 2013). "Production of Sexuals and Mating Frequency in the Stingless Bee Tetragonisca angustula (Latreille) (Hymenoptera, Apidae)". Neotropical Entomology. 42 (5): 474–482. doi:10.1007/s13744-013-0154-0.
- ↑ Izzo, Amanda; Elizabeth, A.; Tibbetts, E. (2012). "Spotting the Top Male: Sexually Selected Signals in Male Polistes dominulus Wasps". Animal Behaviour. 83: 839–845. doi:10.1016/j.anbehav.2012.01.005.
- ↑ Litte, Marcia (1979). "Mischocyttarus flavitarsis in Arizona: Social and Nesting Biology of a Polistine Wasp". Zeitschrift für Tierpsychologie.
- 1 2 Hoglund, J.; Kalais, J. A.; Fiske, P. (1992). "The costs of secondary sexual characters in the lekking great snipe (Gallinago media)". Behavioral Ecology and Sociobiology. 30: 309–315.
- ↑ Alatalo, R. V; Höglund, J.; Lundberg, A (1991). "Lekking in the black grouse: a test of male viability". Nature. 352: 155–156. doi:10.1038/352155a0.
- ↑ Reynolds, J. D. & Gross, M. R. "Costs and Benefits of Female Mate Choice : Is There a Lek Paradox ?" 136, 230–243 (1990).
- ↑ Wickman, P.; Jansson, P. (1997). "An estimate of female mate searching costs in the lekking butterfly Coenonympha pamphilus". Behavioral Ecology and Sociobiology. 40: 321–328. doi:10.1007/s002650050348.
- ↑ Grafe, T. Ulmar (May 1997). "Costs and benefits of mate choice in the lek-breeding reed frog, Hyperolius marmoratus". Animal Behaviour. 53 (5): 1103–1117. doi:10.1006/anbe.1996.0427.
- ↑ Mackenzie, A.; Reynolds, J. D.; Sutherland, W. (1995). "Variation in Male Mating Success on Leks". The American Naturalist. 145: 633–552. doi:10.1086/285759.
- ↑ Miller, Christine; Moore, Allen (2007). "A potential resolution to the lek paradox through indirect genetic effects" (PDF). Proceedings of the Royal Society B: Biological Sciences. 274: 1279–1286. doi:10.1098/rspb.2007.0054. PMC 2176171
 . PMID 17341455.
. PMID 17341455. - 1 2 Kirkpatrick, M (1982). "Sexual Selection and the Evolution of Female Choice". Evolution. 36: 1–12. doi:10.2307/2407961.
- ↑ Kirkpatrick, M.; Ryan, M. (1991). "The evolution of mating preferences and the paradox of the lek". Nature. 350: 33–38. doi:10.1038/350033a0.
- 1 2 3 Tomkins, Joseph L. "Genic capture and resolving the lek paradox". TRENDS in Ecology and Evolution. Vol.19 No.6 June 2004.
- 1 2 Rowe, Locke; Houle, David (1996). "The lek paradox and the capture of genetic variance by condition dependent traits". Proceedings of the Royal Society B: Biological Sciences. 263: 1415–1421. doi:10.1098/rspb.1996.0207. JSTOR 50503.
- 1 2 Zahavi, A (1975). "Mate selection—a selection for a handicap" (PDF). Journal of Theoretical Biology. 53: 205–214. doi:10.1016/0022-5193(75)90111-3. PMID 1195756.
- ↑ Iwasa, Y.; Pomiankowski, A.; Nee, S. (1991). "The Evolution of Costly Mate Preferences II: The 'Handicap' Principle". Evolution. 45: 1431–1442. doi:10.2307/2409890.
- 1 2 Rowe, L.; Houle, D. (1996). "The Lek Paradox and the Capture of Genetic Variance by Condition Dependent Traits". Proceedings: Biological Sciences. 236: 1415–1421.
- 1 2 Hamilton, W. D.; Zuk, M. (1982). "Heritable true fitness and bright birds: A role for parasites?" (PDF). Science. 218: 384–387. doi:10.1126/science.7123238. PMID 7123238.
- ↑ Pomiankowski, A; Moller, A P (1995). "A Resolution of the Lek Paradox". Proceedings of the Royal Society B: Biological Sciences. 260: 21–29. doi:10.1098/rspb.1995.0054.
- 1 2 Foster, M. S.; Beehler, B. M. (1998). "Hotshots, Hotspots, and Female Preferences in the Organization of Lek Mating Systems". The American Naturalist. 131: 203–219.
- 1 2 3 4 5 6 Jiguet, F.; Bretagnolle, V. (2006). "Manipulating lek size and composition using decoys: An experimental investigation of lek evolution models". The American Naturalist. 168: 758–768. doi:10.1086/508808.
- 1 2 Théry, M (1992). "The evolution of leks through female choice: Differential clustering and space utilization in six sympatric manakins". Behavioral Ecology and Sociobiology. 30: 227–237.
- ↑ Clutton-Brock, T. H.; Price, O. F.; Maccou, A. D. C. (1991). "Mate retention, harassment, and the evolution of ungulate leks". Behavioral Ecology. 3: 234–242. doi:10.1093/beheco/3.3.234.
- ↑ Durães, R.; Loiselle, B. A; Blake, J. G. (2008). "Spatial and temporal dynamics at manakin leks: Reconciling lek traditionality with male turnover". Behavioral Ecology and Sociobiology. 62: 1947–1957. doi:10.1007/s00265-008-0626-0.
- ↑ Loiselle BA; Thomas B. Ryder; Renata Durães; Wendy Tori; John G. Blake; Patricia G. Parker (2007). "Kin Selection Does Not Explain Male Aggregation at Leks of 4 Manakin Species". Behavioral Ecology. 18 (2): 287–291. doi:10.1093/beheco/arl081.
- ↑ DB McDonald; WK Potts (1994). "Cooperative display and relatedness among males in a lek-mating bird". Science. 266 (5187): 1030–2. Bibcode:1994Sci...266.1030M. doi:10.1126/science.7973654. PMID 7973654.
- ↑ Hoglund, J (2003). "Lek-kin in birds – Provoking theory and surprising new results" (PDF). Annales Zoologici Fennici. 40: 249–253.
- ↑ Petrie, Marion; Krupa, Andrew; Burke, Terry (1999). "Peacocks lek with relatives even in the absence of social and environmental cues". Nature. 401 (6749): 155–157. Bibcode:1999Natur.401..155P. doi:10.1038/43651.
- ↑ Concannon, Moira R.; Stein, Adam C.; Uy, J. Albert C. (2012). "Kin selection may contribute to lek evolution and trait introgression across an avian hybrid zone". Molecular Ecology. 21 (6): 1477–1486. doi:10.1111/j.1365-294X.2012.05474.x. ISSN 1365-294X.
- ↑ Brumfield, Robb T.; Liu, Liang; Lum, David E.; Edwards, Scott V. (2008-10-01). "Comparison of Species Tree Methods for Reconstructing the Phylogeny of Bearded Manakins (Aves: Pipridae, Manacus) from Multilocus Sequence Data". Systematic Biology. 57 (5): 719–731. doi:10.1080/10635150802422290. ISSN 1063-5157. PMID 18853359.
- ↑ Gibson, Robert M.; Aspbury, Andrea S.; McDaniel, Leonard L. (2002). "Active formation of mixed–species grouse leks: a role for predation in lek evolution?". Proceedings of the Royal Society of London B: Biological Sciences. 269 (1509): 2503–2507. doi:10.1098/rspb.2002.2187. ISSN 0962-8452. PMC 1691199
 . PMID 12573063.
. PMID 12573063.