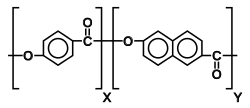
Liquid-crystal polymer
| Solid LCP | |
|---|---|
| Specific Gravity | 1.38 to 1.95 |
| Elasticity modulus (E) | 8530 to 17200 MPa |
| Tensile strength (σt) | 52.8 to 185 MPa |
| Tensile Elongation (%) | 0.26 to 6.2 |
| Notched Izod Impact | 21.0 to 82.5 kJ/m2 |
Liquid-crystal polymers (LCPs) are a class of aromatic polymers. They are extremely unreactive and inert, and highly resistant to fire.
Background
Liquid crystallinity in polymers may occur either by dissolving a polymer in a solvent (lyotropic liquid-crystal polymers) or by heating a polymer above its glass or melting transition point (thermotropic liquid-crystal polymers).[2] Liquid-crystal polymers are present in melted/liquid or solid form.[3] In solid form the main example of lyotropic LCPs is the commercial aramid known as Kevlar. Chemical structure of this aramid consists of linearly substituted aromatic rings linked by amide groups. In a similar way, several series of thermotropic LCPs have been commercially produced by several companies (e.g., Vectran / Ticona).
A high number of LCPs, produced in the 1980s, displayed order in the melt phase analogous to that exhibited by nonpolymeric liquid crystals. Processing of LCPs from liquid-crystal phases (or mesophases) gives rise to fibers and injected materials having high mechanical properties as a consequence of the self-reinforcing properties derived from the macromolecular orientation in the mesophase.
Today, LCPs can be melt-processed on conventional equipment at high speeds with excellent replication of mold details. In fact, the high ease of forming of LCPs is an important competitive advantage against other plastics, as it offsets high raw material cost.[4]
The class of polar and bowlic LCPs, with unique properties and important potential applications, remains to be exploited.[5]
Properties
A unique class of partially crystalline aromatic polyesters based on p-hydroxybenzoic acid and related monomers, liquid-crystal polymers are capable of forming regions of highly ordered structure while in the liquid phase. However, the degree of order is somewhat less than that of a regular solid crystal. Typically LCPs have a high mechanical strength at high temperatures, extreme chemical resistance, inherent flame retardancy, and good weatherability. Liquid-crystal polymers come in a variety of forms from sinterable high temperature to injection moldable compounds. LCP can be welded, though the lines created by welding are a weak point in the resulting product. LCP has a high Z-axis coefficient of thermal expansion.
LCPs are exceptionally inert. They resist stress cracking in the presence of most chemicals at elevated temperatures, including aromatic or halogenated hydrocarbons, strong acids, bases, ketones, and other aggressive industrial substances. Hydrolytic stability in boiling water is excellent. Environments that deteriorate the polymers are high-temperature steam, concentrated sulfuric acid, and boiling caustic materials.
Polar and bowlic LCPs are ferroelectrics, with reaction time order-of-magnitudes smaller than that in conventional LCs and could be used to make ultrafast switches. Bowlic columnar polymers possess long, hollow tubes; with metal or transition metal atoms added into the tube, they could form ultrahigh-Tc superconductors.[6]
Uses
Because of their various properties, LCPs are useful for electrical[7] and mechanical parts, food containers, and any other applications requiring chemical inertness and high strength. LCP is particularly good for microwave frequency electronics due to low relative dielectric constants, low dissipation factors, and commercial availability of laminates. Packaging microelectromechanical systems (MEMS) is another area that LCP has recently gained more attention.
Trade names
LCP is sold by manufacturers under a variety of trade names. These include:
- Kevlar
- Vectran
- Zenite 5145L is a liquid crystal polymer with 45% glass fiber filler, originally developed by DuPont, which is used for injection molded parts with intricate features. Typical uses include electronic packaging, housing. etc. The heat deflection temperature is 290oC. Relative Temperature Index (RTI considering strength but not impact or flexing) is 130oC. The density is about 1.76 g/cm3. The typical tensile strength at room temperature is 0.13 GPa (19 ksi). Melting temperature 319oC. The Deflection Temperature Under Load (DTUL) is 275oC.
Application-
cook ware, microwave oven, microwave oven parts, circuit boards, coiling parts, insulation in small application part, semiconductor handling devices, parts of electric motors and stators,socket, connector, chip carriers and PCBs.
References
- ↑ "Vectran molecular structure". Retrieved 2012-11-22.
- ↑ Shibaev, Valery P.; Lam, Lui, eds. (1994). Liquid Crystalline and Mesomorphic Polymers. New York: Springer.
- ↑ Callister (2007): "Materials Science and Engineering - An Introduction," 557-558.
- ↑ Charles A. Harper, ed., Modern Plastics Handbook, ISBN 0-07-026714-6, 2000.
- ↑ Lam, Lui (1988). "Bowlic and polar liquid crystal polymers". Mol. Cryst. Liq. Cryst. 155, 531.
- ↑ See [2],[5].
- ↑ FCI (2000): "Metral Signal Header 1 Mod, 4 Row Press-Fit", , 8 (note 2)
External links
- Prospector
- Bowlic liquid crystal from San Jose State University