Lyman–Werner photons
A Lyman-Werner photon is an ultraviolet photon with an energy in the range of 11.2 to 13.6 eV, corresponding to the energy range in which the Lyman and Werner absorption bands of molecular hydrogen (H) are found. A photon in this energy range with a frequency that coincides with that of one of the lines in the Lyman or Werner bands can be absorbed by H, placing the molecule in an excited electronic state. Radiative decay from this excited state occurs rapidly, with roughly 15% of these decays occurring into the vibrational continuum of the molecule, resulting in its dissociation.[1] This two-step photodissociation process, known as the Solomon process, is one of the main mechanisms by which molecular hydrogen is destroyed in the interstellar medium.
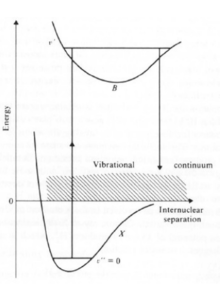
In reference to the figure shown (click on the figure to zoom), Lyman Werner photons are emitted as described below:
- A hydrogen molecule can absorb a far ultra violet photon (11.2eV<energy of the photon<13.6eV) and make a transition from the ground electronic state X to excited state B (Lyman) or C (Werner).
- Radiative decay occurs rapidly.
- 10-15% of the decays occur into the vibrational continuum. This means that the hydrogen molecule has dissociated.
- Photo-dissociation fragments carry away some of the photon energy as kinetic energy, heating the gas.
- Rest of the decays are either radiative decay (Infra-Red emission) or collisional which ultimately end up heating the gas.
References
- ↑ Draine, Bruce T.; Bertoldi, Frank (1996). "Structure of Stationary Photodissociation Fronts". Astrophysical Journal. 468: 269. arXiv:astro-ph/9603032
 . Bibcode:1996ApJ...468..269D. doi:10.1086/177689.
. Bibcode:1996ApJ...468..269D. doi:10.1086/177689.