Magnetic resonance (quantum mechanics)
Magnetic resonance is a phenomenon that affects a Magnetic dipole when placed in a uniform static magnetic field. Its energy is split into a finite number of energy levels, depending on the value of quantum number of angular momentum. This is similar to energy quantization for atoms, say
e−
in H atom; in this case the atom, in interaction to an external electric field, transitions between different energy levels by absorbing or emitting photons. Similarly if a magnetic dipole is perturbed with electromagnetic field of proper frequency(), it can transit between its energy eigenstates, but as the separation between energy eigenvalues is small, the frequency of the photon will be the microwave or radio frequency range. If the dipole is tickled with a field of another frequency, it is unlikely to transition. This phenomenon is similar to that, when a system is acted on by a periodic force of frequency equal to its natural frequency.
Quantum mechanical explanation
As a magnetic dipole, using a spin system such as a proton; according to the quantum mechanical state of the system, denoted by : , evolved by the action of an unitary operator ; the result obeys Schrödinger equation:
States with definite energy evolve in time with phase ,( ) where E is the energy of the state, since the probability of finding the system in state = is independent of time. Such states are termed stationary states, so if a system is prepared in a stationary state, (i.e. one of the eigenstates of the Hamiltonian operator), then P(t)=1,i.e. it remains in that state indefinitely. This the case only for isolated systems. When a system in a stationary state is perturbed, its state changes, so it is no longer an eigenstate of the system's complete Hamiltonian. This same phenomenon happens in magnetic resonance for a spin system in a magnetic field.
The Hamiltonian for a magnetic dipole (associated with a spin particle) in a magnetic field is:
Here is the larmor precession frequency of the dipole for magnetic field and is z Pauli matrix. So the eigenvalues of are and . If the system is perturbed by a weak magnetic field , rotating counterclockwise in x-y plane (normal to ) with angular frequency , so that , then and are not eigenstates of the Hamiltonian, which is modified into
It is inconvenient to deal with a time-dependent hamiltonian. To make time-independent requires a new reference frame rotating with ,i.e. rotation operator on , which amounts to basis change in Hilbert space. Using this on Schrödinger's equation, the Hamiltonian becomes:
Writing in the basis of as-
Using this form of the Hamiltonian a new basis is found:

where and
This Hamiltonian is exactly similar to that of a two state system with unperturbed energies & with a perturbation expressed by ; According to Rabi oscillation, starting with state, a dipole in parallel to with energy , the probability that it will transit to state (i.e. it will flip) is
Now if , i.e. rotates with larmor frequency of the dipole in magnetic field, then at some point of time, say , it flips into another (previous) energy eigenstate ; i.e. a diplole can be completely flipped. When , the probability of change of energy state is small, so the previous case reflected resonance. This resonance condition is used for measurement of the magnetic moment of a dipole, magnetic field at a point in space, etc.
A special case to show applications
A special case occurs where a system oscillates between two unstable levels that have the same life time .[1] If atoms are excited at a constant, say n/time, to the first state, some decay and the rest have a probability to transition to the second state, so in the time interval between t and (t+dt) the number of atoms that jump to the second state from the first is , so at time t the number of atoms in the second state is
=
The rate of decay from state two depends on the number of atoms that were collected in that state from all previous intervals, so the number of atoms in state 2 is ; The rate of decay of atoms from state two is proportional to the number of atoms present in that state, while the constant of proportionality is decay constant . Performing the integration rate of decay of atoms from state two is obtained as:
From this expression many interesting points can be exploited, such
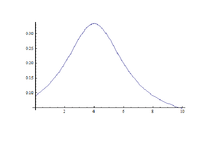
- Varying uniform magnetic field so that in produces a Lorentz curve(see Cauchy distribution), detecting the peak of that curve, the abscissa of it gives , so now (angular frequency of rotation of = , so from the known value of and , the gyromagnetic ratio of the dipole can be measured; by this method we can measure Nuclear spin where all electronic spins are balanced. Correct measurement of nuclear magnetic moment helps to understand the character of nuclear force.
- If is known, by varying , the value of can be obtained. This measurement technique is precise enough for use in sensitive magnetometers. Using this technique, the value of magnetic field acting at a particular lattice site by its environment inside a crystal can be obtained.
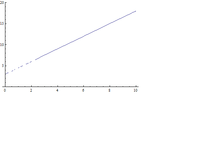
- By measuring half-width of the curve, d=, for several values of (i.e. of ), we can plot d vs , and by extrapolating this line for , the lifetime of unstable states can be obtained from the intercept.
Rabi's method

The existence of spin angular momentum of electrons was discovered experimentally by the Stern–Gerlach experiment. In that study a beam of neutral atoms with one electron in the valence shell, carrying no orbital momentum (from the viewpoint of quantum mechanics) was passed through an inhomogeneous magnetic field. This process was not approximate due to the small deflection angle, resulting in considerable uncertainty in the measured value of the split beam.
Rabi's method was an improvement over Stern-Gerlach. As shown in the figure, the source emits a beam of neutral atoms, having spin angular momentum . The beam passes through a series of three aligned magnets. Magnet 1 produces an inhomogeneous magnetic field with a high gradient(as in Stern-Gerlach), so the atoms having 'upward' spin (with ) will deviate downward (path 1),i.e. to the region of less magnetic field B, to minimize energy. Atoms with 'downward' spin with ) will deviate upward similarly (path 2). Beams are passed through slit 1, to reduce any effects of source beyond. Magnet 2 produces only a uniform magnetic field in the vertical direction applying no force on the atomic beam, and magnet 3 is actually inverted magnet 1. In the region between the poles of magnet 3, atoms having 'upward' spin get upward push and atoms having 'downward' spin feel downward push, so their path remains 1 and 2 respectively. These beams pass through a second slit S2, and arrive at detector and get detected.
If a horizontal rotating field ,angular frequency of rotation is applied in the region between poles of magnet 2, produced by oscillating current in circular coils then there is a probability for the atoms passing through there from one spin state to another ( and vice versa), when =, Larmor frequency of precession of magnetic moment in B. The atoms that transition from 'upward' to 'downward' spin will experience a downward force while passing through magnet 3, and will follow path 1'. Similarly, atoms that change from 'downward' to 'upward' spin will follow path 2', and these atoms will not reach the detector, causing a minimum in detector count. If angular frequency of is varied continuously, then a minimum in detector current will be obtained (when =). From this known value of (, where g is 'Landé g factor'), 'Landé g factor' is obtained which will enable one to have correct value of magnetic moment . This experiment, performed by Isidor Isaac Rabi is more sensitive and accurate compared than Stern-Gerlach.
Correspondence between classical and quantum mechanical explanations
Though the notion of spin angular momentum arises only in quantum mechanics and has no classical analogue, magnetic resonance phenomena can be explained via classical physics to some extent. When viewed from the reference frame attached to the rotating field, it seems that the magnetic dipole precesses around a net magnetic field , where is the unit vector along uniform magnetic field and is the same in the direction of rotating field and .
Proof of Classical Expression for Precession 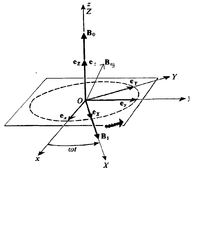 Pictorial representation of classical Larmor precession
Pictorial representation of classical Larmor precessionAs Classical Electrodynamics tells that torque on a magnetic dipole of moment is × ,so its equation of motion is
× , (where is the angular momentum associated with dipole) so -
- ×
For considered case dipole is under the action of magnetic field and , hence
× It is easier to solve it by transforming co-ordinate system to OXYZ in which becomes OX axis, in that frame -
×
here Using and , one can see that-
- ×× ( )
so, here effective field becomes :
So when , a high precession amplitude allows the magnetic moment to be completely flipped. Classical and quantum mechanical predictions correspond well, which can be viewed as an example of the Bohr Correspondence principle, which states that quantum mechanical phenomena, when predicted in classical regime, should match the classical result. The origin of this correspondence is that the evolution of the expected value of magnetic moment is identical to that obtained by classical reasoning. The expectation value of the magnetic moment is . The time evolution of is given by
so,
So, and
which looks exactly similar to the equation of motion of magnetic moment in classical mechanics -
This analogy in the mathematical equation for the evolution of magnetic moment and its expectation value facilitates to understand the phenomena without a background of quantum mechanics.
Magnetic resonance imaging
In magnetic resonance imaging (MRI) the proton's spin angular momentum is used. The most available source for protons is the hydrogen atoms in water. A strong magnetic field applied to water causes a splitting of energy associated with spin angular momentum, -() and -(), using .
According to the Boltzmann distribution (the number of systems having energy out of at temperature ) is (where k is Boltzmann constant) lower energy level, associated with spin is more populated than the other. In the presence of a rotating magnetic field more protons flip from to than flip the other way, causing absorption of microwave or radio-wave radiation (from the rotating field) . When the field is withdrawn, protons tend to reequilibrate along the Boltzmann distribution, so some protons transition from higher energy level to lower ones, emitting microwave or radio-wave radiation at specific frequencies.
Instead of nuclear spin, spin angular momentum of unpaired electrons is used in EPR (Electron paramagnetic resonance) to detect free radicals, etc.
Magnetic resonance as a quantum phenomenon
The phenomenon of magnetic resonance is rooted in the existence of spin angular momentum of a quantum system and its specific orientation with respect to an applied magnetic field. Both cases have no explanation in the classical approach and can be understood only by using quantum mechanics. Some people claim that purely quantum phenomena are those that cannot be explained by the classical approach. For example, phenomena in the microscopic domain that can to some extent be described by classical analogy are not really quantum phenomena. Since the basic elements of magnetic resonance have no classical origin, although analogy can be made with Classical Larmor precession, MR should be treated as a quantum phenomenon.
See also
References
- ↑ Page-449, Quantum Mechanics, Vol.1, Claude Cohen-Tannoudji, Bernard Diu, Frank Laloe
- Feynman, Leighton, Sands. The Feynman Lectures on Physics, Volume 3. Narosa Publishing House, New Delhi, 2008.
- Cohen-Tannoudji Claude. Quantum Mechanics. Wiley-VCH.
- Griffiths David J. An Introduction to Quantum Mechanics. Pearson Education,Inc.