Malvalic acid
| | |
| Names | |
|---|---|
| IUPAC name
7-(2-octyl-1-cyclopropenyl)heptanoic acid | |
| Identifiers | |
| 503-05-9 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:6673 |
| ChemSpider | 9987 |
| KEGG | C08321 |
| PubChem | 10416 |
| |
| |
| Properties | |
| C18H32O2 | |
| Molar mass | 280.44548 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Malvalic acid is a cyclopropenic fatty acid found in cottonseed oil. The cyclopropene ring is thought to be one of the causes of abnormalities that develop in animals that ingest cottonseed oil.[1] This reactivity could be cause for concern depending on concentration. Hydrogenation of the oil destroys malvalic acid.
Biosynthesis
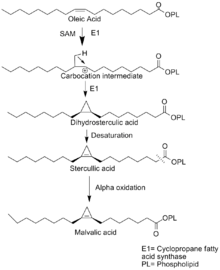
The biosynthesis of malvalic acid starts when the electrophilic addition of methylene occurs via SAM the alkylating agent. That electrophilic addition gives a raise to carbocation intermediate. At this point, the cation discharged by formation of cyclopropane ring and loss of proton. The Enzyme cyclopropane fatty acid synthesis catalyzed the reaction to form dihydrosterculic acid, which will be dehydrogenated to sterculic acid. Finally, sterculic acid will go over chain shortening by α-Oxidation in which carboxyl carbon would be removed to form malvalic acid.[2]
History
Wilson et al.[3] demonstrated the co-occurrence of malvalic acid and the corresponding cyclopropane acids in several types of seeds. He suggested that methylene addition to oleic acid gave rise to dihydrosterculic acid, which was desaturated to sterculic acid, and that 8-heptadecenoic acid was similarly the precursor of dihydromalvalic acid and malvalic acid. Smith and Bu'Lock[4] showed that in Hibiscus seedlings the chains of sterculic and malvalic acids, but not the ring methylene carbon, were derived from acetate. They showed that the labeling pattern in malvalic acid was the same as that in sterculic acid minus the carboxyl carbon. They explained the shortening by α-oxidation occurring during the biogenesis of malvalic acid. Hooper and Law[5] demonstrated that the ring methylene carbon of both cyclopropane and cyclopropene acids was derived from the methyl group of methionine in Hibiscus, and suggested from the distribution of label that the pathway was Oleic ⇒ dihydrosterculic ⇒ sterculic acid.[6]
See also
References
- ↑ Hornback, Joseph (2006). Organic Chemistry, Second Edition. Cengage Learning. p. 207. ISBN 978-0534389512.
- ↑ Dewick, Paul (2009). Medicinal Natural products. pp. 46–55. ISBN 9780470741689.
- ↑ Wilson, T. L.; Smith, C. R.; Mikolajczak, K. L. (1961). "Characterization of cyclopropenoid acids in selected seed oils". Journal of the American Oil Chemists Society. 38 (12): 696. doi:10.1007/BF02633058.
- ↑ Smith, G. N.; Bu'Lock, J. D. (1964). "Biogenesis of cyclopropene acids". Biochemical and Biophysical Research Communications. 17 (4): 433. doi:10.1016/0006-291X(64)90025-7.
- ↑ Hooper, N. K.; Law, J. H. (1965). "Biosynthesis of cyclopropane compounds VII. Synthesis of cyclopropane and cyclopropene fatty acids by seedlings". Biochemical and Biophysical Research Communications. 18 (3): 426. doi:10.1016/0006-291X(65)90725-4.
- ↑ Yano, I.; Morris, L. J.; Nichols, B. W.; Jams, A. T. (1972). "The biosynthesis of cyclopropane and cyclopropene fatty acids in higher plants (Malvaceae)". Lipids. 7: 35. doi:10.1007/BF02531267.