Mechanostat
The Mechanostat is a model describing bone growth and bone loss. It was promoted by Harold Frost and described extensively in the Utah Paradigm of Skeletal Physiology[1][2][3][4][5] in the 1960s. The Mechanostat is a refinement of Wolff's law described by Julius Wolff (1836–1902).
According to the Mechanostat, bone growth and bone loss is stimulated by the local mechanical elastic deformation of bone. The reason for the elastic deformation of bone is the peak forces caused by muscles (e.g. measurable using mechanography). The Adaptation (feed-back control loop) of bone according to the maximum forces is considered to be a lifelong process. Hence bone adapts its mechanical properties according to the needed mechanical function – bone mass, bone geometry and hence bone strength (see also Stress-strain index, SSI) is adapted according to the everyday usage / needs.
Due to this control loop, there is a linear relationship in the healthy body between muscle cross sectional area (as a surrogate for typical maximum forces the muscle is able to produce under physiological conditions) and the bone cross sectional area (as a surrogate for bone strength).[6][7]
These relations are of immense importance especially for bone loss situations like in osteoporosis, since an adapted training utilizing the needed maximum forces on the bone can be used to stimulate bone growth and hence prevent or help to minimize bone loss. An example for such an efficient training is vibration training or whole body vibration.
Modeling and remodeling
Frost defined four regions of elastic bone deformation which result in different consequences on the control loop:
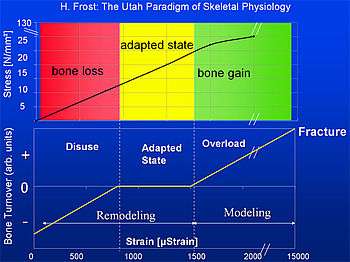
- Disuse:
Strain < circa 800μStrain: Remodeling (bone adaptation and bone repair) Bone mass and bone strength is reduced. - Adapted State:
strain between ca. 800μStrain and ca. 1500μStrain: Remodeling (bone repair) Bone mass and bone strength stays constant (homeostasis: bone resorption=bone formation) - Overload:
Strain > circa 1500μStrain: Modeling (bone growth) bone mass and bone strength is increased - Fracture:
Strain > circa 15000μStrain: maximum elastically deformation exceeded - bone fracture.
According to this a typical bone, e.g. the tibia has a security margin of about 5 to 7 between typical load (2000 to 3000 μStrain) and fracture load (about 15000μStrain).
Unit: Strain E
The elastic deformation of bone is measured in μStrain.[2][3] 1000μStrain = 0.1% change of length of the bone.
- Strain E at length l and change of length Δl:
It has to be considered that bone strength is highly dependent on geometry and direction of the acting forces in relation to this geometry. The fracture load for axial forces of the tibia for example is about 50 to 60 times the body weight. The fracture load for forces perpendicular to the axial direction is about 10 times lower.
Different type of bones can have different modelling and remodelling thresholds. The modeling threshold of the tibia is about 1500 μStrain (0.15% change of length), the modelling threshold for bone of the skull is lowered by the factor of 6 to 8. Since the physical material properties of bone (as a material) are not altered in the different bone types of the body, this difference in modelling threshold results in an increased bone mass and bone strength and hence in an increased safety factor (relation between fracture load and typical loads) for the skull compared to the tibia. A lower modeling threshold means that the same typical daily forces result in a ‘thicker’ and hence stronger bone at the skull.
Examples
Typical examples of the influence of maximum forces and the resulting elastic deformations on bone growth or bone loss are extended flights of astronauts and cosmonauts as well as patient with paraplegia due to an accident. For example, a patient in a wheel chair who is using his arms but due to his paraplegia not his legs will suffer massive muscle and bone loss only in his legs due to the lack of usage of the legs. However the muscles and bones of the arms which are used every day will stay the same or might even be increased depending on the usage.[8]
The same effect can be observed for long flight Astronauts or Cosmonauts.[9] While they still use their arms in an almost normal manner due to the lack of gravity in space there are no maximum forces induced on the bones of the legs.
Harold Frost applied the Mechanostat model not only to skeletal tissues but also to fibrous collagenous connective tissues, such as ligaments, tendons and fascia.[10][11] He described their adaptational responsiveness to strain in his "stretch-hypertrophy rule":
- "Intermittent stretch causes collagenous tissues to hypertrophy until the resulting increase in strength reduces elongation in tension to some minimum level".[12]
Similar to the responsiveness of bony tissues this adaptational response occurs only if the mechanical strain exceeds a certain threshold value. Harold Frost proposed that for dense collagenous connective tissues the related threshold value is around 4% strain elongation.[13]
Literature
- ↑ Frost H.M.: Defining Osteopenias and Osteoporoses: Another View (With Insights From a New Paradigm), Bone 1997, Vol. 20, No. 5, 385-391, PMID 9145234
- 1 2 Frost H.M.: The Utah Paradigm of Skeletal Physiology Vol. 1, ISMNI, 1960
- 1 2 Frost H.M.: The Utah Paradigm of Skeletal Physiology Vol. 2, ISMNI, 1960
- ↑ Frost H.M.: The Utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs, J Bone Miner Metab. 2000; 18:305–316, PMID 11052462
- ↑ Frost H.M., Schoenau E.: The muscle-bone unit in children and adolescents: an overview, 2000, J. Pediatr Endorcrinol Metab 13:571-590, PMID 10905381
- ↑ Schoenau E., NeuC.M., Beck B., Manz F., Rauch F.: Bone Mineral content per Muscle Cross-Sectional Area as an Index of the Functional Muscle-Bone Unit, J Bone Mineral Res, Vol.17, S.1095-1101, 2002, PMID 12054165
- ↑ Schießl H., Frost H.M., Jee W.S.S.: Estrogen and BoneMuscle Strength and Mass Relationships, Bone, Vol.22, S.1-6, 1998, PMID 9437507
- ↑ Eser P. et al.: Relationship between duration of paralysis and bone structure: a pQCT Study of spinal cord injured individuals, Bone, Vol.34, S.869-880, 2004, PMID 15121019
- ↑ Blottner D., Salanova M., Püttmann B., Schiffl G., Felsenberg D., Buehring B., Rittweger J.: Human skeletal muscle structure and function preserved by vibration muscle exercise following 55 days of bed rest, Eur J. Appl Physiol, 2006, Vol. 97, S. 261-271, doi:10.1007/s00421-006-0160-6 PMID 16568340
- ↑ Frost, Harold "New targets for fascial, ligament and tendon research: A perspective from the Utah paradigm of skeletal physiology" J Musculoskel Neuron Interact 2003; 3(3):201-209
- ↑ Frost, Harold "The physiology of cartilagenous, fibrous, and bony tissue. C.C. Thomas, 1972
- ↑ Frost, Harold "The physiology of cartilagenous, fibrous, and bony tissue. C.C. Thomas, 1972, page 176
- ↑ Frost, Harold "Does the anterior cruciate have a modeling threshold? A case for the affirmative". J Musculoskel Neuron Interact 2001; 2(2):131-136
External links
- ISMNI - International Society of Musculoskeletal and Neuronal Interactions