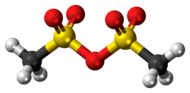
Methanesulfonic anhydride
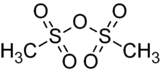 | |
 | |
| Names | |
|---|---|
| IUPAC name
methylsulfonyl methanesulfonate | |
| Other names
methanesulfonic acid methylsulfonyl ester | |
| Identifiers | |
| 7143-01-3 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 73591 |
| ECHA InfoCard | 100.027.675 |
| PubChem | 81560 |
| |
| |
| Properties | |
| C2H6O5S2 | |
| Molar mass | 174.20 g/mol |
| Appearance | White solid |
| Density | 0.92 g/ml[1] |
| Melting point | 69.5–70 °C (157.1–158.0 °F; 342.6–343.1 K)[2] |
| Hydrolyses | |
| Solubility | Soluble in most aprotic organic solvents |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Methanesulfonic anhydride is the acid anhydride of methanesulfonic acid. Like methanesulfonyl chloride, it may be used to generate mesylates (methanesulfonyl esters). It is commercially available but may also be prepared by the reaction of phosphorus pentoxide with methanesulfonic acid at 80 °C.[2]
2 P2O5 + 6 CH3SO3H → 3 (CH3SO2)2O + 4 H3PO4
See also
References
- ↑ Wachtmeister, C. A.; Pring, B.; Osterman, Siv; Ehrenberg, L.; Brunvoll, J.; Bunnenberg, E.; Djerassi, Carl; Records, Ruth (1966). "The Synthesis of Some Tritium-labelled Mutagenic Alkyl Alkanesulfonates.". Acta Chemica Scandinavica. 20: 908–910. doi:10.3891/acta.chem.scand.20-0908.
- 1 2 Field, Lamar; Settlage, Paul H. (March 1954). "Alkanesulfonic Acid Anhydrides". Journal of the American Chemical Society. 76 (5): 1222–1225. doi:10.1021/ja01634a005.
This article is issued from Wikipedia - version of the 7/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.